Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-08-18 , DOI: 10.1016/j.bioorg.2018.08.010 Majid Nazir , Muhammad Athar Abbasi , Aziz-ur-Rehman , Sabahat Zahra Siddiqui , Khalid Mohammed Khan , Kanwal , Uzma Salar , Muhammad Shahid , Muhammad Ashraf , Muhammad Arif Lodhi , Farman Ali Khan
|
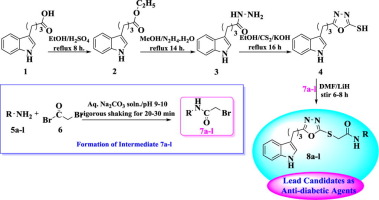
Current study is based on the sequential conversion of indolyl butanoic acid (1) into ethyl indolyl butanoate (2), indolyl butanohydrazide (3), and 1,3,4-oxadiazole-2-thiol analogs (4) by adopting chemical transformations. In a parallel series of reactions, 2-bromo-N-phenyl/arylacetamides (7a-l) were synthesized by reacting different amines derivatives (5a-l) with 2-bromoacetyl bromide (6) to serve as electrophile. Then, the synthesized electrophiles (7a-l) were treated with nucleophilic 1,3,4-oxadiazole-2-thiol analog (4) to afford a range of N-substituted derivatives (8a-l). The structural confirmation of all the synthetic compounds was carried out by IR, 1H-, 13C NMR, EI-MS, and CHN analysis data. All synthesized molecules (8a-l) were tested for their antidiabetic potential via inhibition of the α-glucosidase enzyme followed by their in silico study. Their cytotoxicity profile was also ascertained via hemolytic activity and all of them possessed very low cytotoxicity. Compounds 8h and 8l were found most active having IC50 values 9.46 ± 0.03 µM and 9.37 ± 0.03 µM, respectively. However, all other molecules also exhibited good to moderate inhibition potential with IC50 values between 12.68 ± 0.04–37.82 ± 0.07, compared to standard acarbose (IC50 = 37.38 ± 0.12 µM), hence can be used as lead molecules for further research in order to get better antidiabetic agents.
中文翻译:

新的基于吲哚的杂合恶二唑支架与N-取代的乙酰胺:作为有效的抗糖尿病药
当前的研究基于通过化学转化将吲哚基丁酸(1)依次转化为吲哚基丁酸乙酯(2),吲哚基丁酰肼(3)和1,3,4-恶二唑-2-硫醇类似物(4)。在一系列平行的反应中,通过使不同的胺衍生物(5a-1)与2-溴乙酰基溴(6)反应用作亲电试剂来合成2-溴-N-苯基/芳基乙酰胺(7a-1)。然后,用亲核的1,3,4-恶二唑-2-硫醇类似物(4)处理合成的亲电试剂(7a-1),得到一系列N-取代的衍生物(8a-1)。通过IR,1 H-,13 C NMR,EI-MS和CHN分析数据进行所有合成化合物的结构确认。通过抑制α-葡萄糖苷酶,然后进行计算机模拟研究,测试了所有合成分子(8a-1)的抗糖尿病潜力。还通过溶血活性确定了它们的细胞毒性谱,并且它们都具有非常低的细胞毒性。发现化合物8h和8l最具有IC 50活性值分别为9.46±0.03 µM和9.37±0.03 µM。但是,与标准阿卡波糖(IC 50 = 37.38±0.12 µM)相比,所有其他分子也表现出良好至中度的抑制潜力,IC 50值在12.68±0.04–37.82±0.07之间,因此可以用作进一步研究的先导分子。为了获得更好的抗糖尿病药。









































 京公网安备 11010802027423号
京公网安备 11010802027423号