当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The activity and action mechanism of novel short selective LL‐37‐derived anticancer peptides against clinical isolates of Escherichia coli
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-03 , DOI: 10.1111/cbdd.13381 Hossein Aghazadeh 1 , Hamed Memariani 1 , Reza Ranjbar 2 , Kamran Pooshang Bagheri 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-03 , DOI: 10.1111/cbdd.13381 Hossein Aghazadeh 1 , Hamed Memariani 1 , Reza Ranjbar 2 , Kamran Pooshang Bagheri 1
Affiliation
|
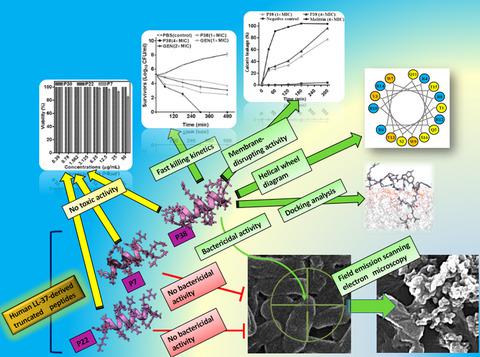
Human cathelicidin LL‐37 has recently attracted interest as a potential therapeutic agent, mostly because of its ability to kill a wide variety of pathogens and cancer cells. In this study, we aimed to investigate the antibacterial activity and cytotoxicity of previously designed LL‐37 anticancer derivatives (i.e., P7, P22, and P38). Calcein release assay and field emission‐scanning electron microscopy (FE‐SEM) were performed to elucidate the possible mechanism of action of P38, the peptide with the highest bactericidal activity. In silico analysis demonstrated the amphipathic alpha‐helical structure for three peptides. Antibacterial activity of P38 against multidrug‐resistant (MDR) clinical isolates of Escherichia coli was higher than that of P7 and P22. P38 caused no hemolysis or cytotoxicity. Treating calcein‐loaded E. coli with 4× MIC of P38 resulted in more than 96% leakage of calcein. Noticeably, FE‐SEM revealed that P38 killed E. coli by disrupting the bacterial membrane. Molecular docking studies showed that P38 had a much higher affinity for the outer membrane of Gram‐negative bacteria compared with both P22 and P7. Owing to the bactericidal activity of P38 against MDR E. coli isolates and its negligible cytotoxicity, P38 has the potential for further studies in a mouse model of infectious disease.
中文翻译:

新型短选择性LL-37衍生抗癌肽对大肠杆菌临床分离株的活性和作用机制
人Cathelicidin LL-37最近作为一种潜在的治疗剂引起了人们的兴趣,主要是因为它具有杀死多种病原体和癌细胞的能力。在这项研究中,我们旨在研究先前设计的LL-37抗癌衍生物(即P7,P22和P38)的抗菌活性和细胞毒性。进行了钙黄绿素释放测定和场发射扫描电子显微镜(FE-SEM),以阐明具有最高杀菌活性的肽P38的可能作用机理。在计算机分析中证实了三种肽的两亲性α-螺旋结构。P38对大肠杆菌多药耐药(MDR)临床分离株的抗菌活性高于P7和P22。P38没有引起溶血或细胞毒性。用P38的4倍MIC处理含钙黄绿素的大肠杆菌时,钙黄绿素的泄漏量超过96%。值得注意的是,FE‐SEM显示P38通过破坏细菌膜杀死了大肠杆菌。分子对接研究表明,与P22和P7相比,P38对革兰氏阴性细菌的外膜具有更高的亲和力。由于P38对MDR大肠杆菌分离物具有杀菌活性,并且其细胞毒性可忽略不计,因此P38在小鼠传染病模型中具有进一步研究的潜力。
更新日期:2018-10-03
中文翻译:

新型短选择性LL-37衍生抗癌肽对大肠杆菌临床分离株的活性和作用机制
人Cathelicidin LL-37最近作为一种潜在的治疗剂引起了人们的兴趣,主要是因为它具有杀死多种病原体和癌细胞的能力。在这项研究中,我们旨在研究先前设计的LL-37抗癌衍生物(即P7,P22和P38)的抗菌活性和细胞毒性。进行了钙黄绿素释放测定和场发射扫描电子显微镜(FE-SEM),以阐明具有最高杀菌活性的肽P38的可能作用机理。在计算机分析中证实了三种肽的两亲性α-螺旋结构。P38对大肠杆菌多药耐药(MDR)临床分离株的抗菌活性高于P7和P22。P38没有引起溶血或细胞毒性。用P38的4倍MIC处理含钙黄绿素的大肠杆菌时,钙黄绿素的泄漏量超过96%。值得注意的是,FE‐SEM显示P38通过破坏细菌膜杀死了大肠杆菌。分子对接研究表明,与P22和P7相比,P38对革兰氏阴性细菌的外膜具有更高的亲和力。由于P38对MDR大肠杆菌分离物具有杀菌活性,并且其细胞毒性可忽略不计,因此P38在小鼠传染病模型中具有进一步研究的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号