Science of the Total Environment ( IF 8.2 ) Pub Date : 2018-08-18 , DOI: 10.1016/j.scitotenv.2018.08.239 Nathaniel D. Fletcher , Heather C. Lieb , Katherine M. Mullaugh
|
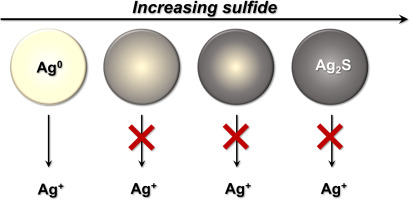
The adoption of silver nanoparticles in consumer goods has raised concerns about the potential environmental harm of their widespread use. We studied chemical transformations that Ag NPs may undergo as they pass through sulfide-rich conditions common in waste water treatment plants (WWTPs), which may limit the release of Ag+ from Ag NPs due to the formation of low-solubility silver sulfide (Ag2S). However, it is uncertain whether sulfidation is complete and if sulfidized Ag NPs continue to release Ag+. To address these uncertainties, we monitored the reaction of Ag NPs with various levels of sulfide with an ion selective electrode and UV/visible spectrophotometry over the course of two months. We characterized the products of the sulfidation reactions with a purge-and-trap acid volatile sulfide (AVS) analysis, which served as a measure of the stability of the sulfidized products because sulfide would be readily lost to oxidation unless it is stabilized as Ag2S. The Ag NP surface plasmon resonance (SPR) absorbance peak was initially diminished and then returned over the course of several days after reaction with limited amounts of sulfide, suggesting a dynamic system that may retain some characteristics of the pristine Ag NPs. However, ICP-MS analysis of sulfidized Ag NP suspensions over a two-month period demonstrates that sulfidation limits the release of Ag+ ions from nanosilver that pass through a WWTP, even when sulfide concentrations are limited relative to silver.
中文翻译:

银纳米颗粒硫化产物的稳定性
在消费品中采用银纳米颗粒引起了人们对其广泛使用的潜在环境危害的担忧。我们研究了化学转化即当它们通过共同的富硫化物条件的Ag纳米颗粒可经历废水处理厂(污水处理厂),其可以Ag的释放限制+选自Ag纳米颗粒由于低溶解度硫化银的形成(银2秒)。但是,尚不确定硫化是否完成以及硫化的Ag NP是否继续释放Ag + 。为了解决这些不确定性,我们通过离子选择性电极和紫外/可见光监测了Ag NPs与不同含量的硫化物的反应分光光度法在两个月的过程中。我们使用吹扫捕集式酸挥发性硫化物(AVS)分析来表征硫化反应的产物,该分析可用来衡量硫化产物的稳定性,因为除非将其硫化物稳定为Ag 2,否则硫化物很容易失去氧化作用S. Ag NP表面等离振子共振(SPR)吸光度峰最初减小,然后在与有限量的硫化物反应后的几天内返回,这表明该动力学系统可以保留原始Ag NP的某些特征。但是,ICP-MS在两个月的时间内对硫化的Ag NP悬浮液的分析表明,硫化会限制Ag +的释放 即使硫化物相对于银的浓度受到限制,来自纳米银的离子也会通过污水处理厂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号