Nature Reviews Chemistry ( IF 38.1 ) Pub Date : 2018-08-17 , DOI: 10.1038/s41570-018-0029-3 Anja Hemschemeier , Thomas Happe
|
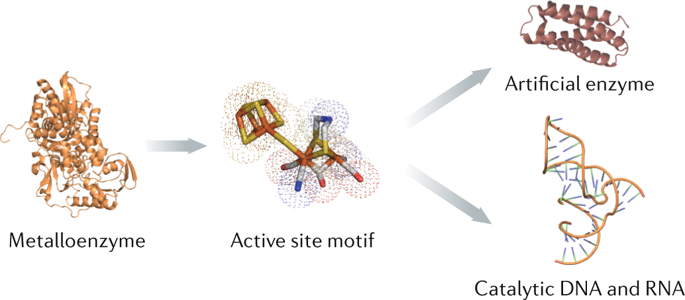
Metal cofactors considerably widen the catalytic space of naturally occurring enzymes whose specific and enantioselective catalytic activity constitutes a blueprint for economically relevant chemical syntheses. To optimize natural enzymes and uncover novel reactivity, we need a detailed understanding of cofactor–protein interactions, which can be challenging to obtain in the case of enzymes with sophisticated cofactors. As a case study, we summarize recent research on the [FeFe]-hydrogenases, which interconvert protons, electrons and dihydrogen at a unique iron-based active site. We can now chemically synthesize the complex cofactor and incorporate it into an apo-protein to afford functional enzymes. By varying both the cofactor and the polypeptide components, we have obtained detailed knowledge on what is required for a metal cluster to process H2. In parallel, the design of artificial proteins and catalytically active nucleic acids are advancing rapidly. In this Perspective, we introduce these fields and outline how chemists and biologists can use this knowledge to develop novel tailored semisynthetic catalysts.
中文翻译:

氧化还原辅助因子的可塑性:从金属酶到氧化还原活性DNA
金属辅因子大大拓宽了天然酶的催化空间,这些酶的特异性和对映选择性催化活性构成了经济相关化学合成的蓝图。为了优化天然酶并发现新的反应性,我们需要对辅因子-蛋白质相互作用的详细了解,这对于具有复杂辅因子的酶而言可能是一个挑战。作为案例研究,我们总结了[FeFe]氢化酶的最新研究,该酶在一个独特的基于铁的活性位点互变质子,电子和二氢。现在,我们可以化学合成复杂的辅因子,并将其整合到载脂蛋白中以提供功能性酶。通过同时改变辅因子和多肽成分,我们获得了有关金属簇处理H所需的详细知识2。同时,人工蛋白质和催化活性核酸的设计也在迅速发展。在本《观点》中,我们介绍了这些领域,并概述了化学家和生物学家如何利用这些知识来开发新颖的量身定制的半合成催化剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号