当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tailoring of carbon nanotubes for the adsorption of heavy metal ions: molecular dynamics and experimental investigations†
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2018-08-16 00:00:00 , DOI: 10.1039/c8me00039e P. Sahu 1, 2, 3, 4, 5 , A. K. Singha Deb 1, 2, 3, 4, 5 , S. K. M. Ali 1, 2, 3, 4, 5 , K. T. Shenoy 1, 2, 3, 4, 5 , S. Mohan 1, 2, 3, 4, 5
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2018-08-16 00:00:00 , DOI: 10.1039/c8me00039e P. Sahu 1, 2, 3, 4, 5 , A. K. Singha Deb 1, 2, 3, 4, 5 , S. K. M. Ali 1, 2, 3, 4, 5 , K. T. Shenoy 1, 2, 3, 4, 5 , S. Mohan 1, 2, 3, 4, 5
Affiliation
|
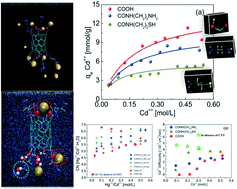
Polluted water sources due to enormously enhanced human population or rapid industrialization need to be treated as they have an adverse effect on human health. CNTs are becoming quite popular in the field of adsorption, which on functionalization with various functional groups can be employed as effective adsorbents. The present study explores the adsorption mechanism of Cd++/Hg++ with carboxyl (COOH), amine (CONH(CH2)2NH2) and sulfur (CONH(CH2)2SH) based CNTs, using molecular dynamics (MD) simulations. The study investigates the molecular level events of adsorption both in aqueous and acidic environments. The MD studies capture well the experimentally observed Langmuir type adsorption isotherms of metal ions for the CNTs. The presence of acid was found to reduce the adsorption of metal ions due to competition with H3O+ ions. The results establish the use of amine based CNTs over sulfur based CNTs for the adsorption of Cd++ ions. However, for the adsorption of Hg++, both amine based CNTs as well as sulfur based CNTs were found to be equally suitable. The simulation trajectories visualize the binding sites for adsorbed ions, which preferentially reside near the O atom for most of the functional groups and also near the N atom of the amine at higher concentration. In addition, the adsorption correlation function and corresponding residence time distribution were used to determine the strength of binding. Also, the rich structural and dynamical events were explored via RDF, CNs and diffusion coefficients. The higher the adsorption capacity of the functionalized CNTs, the lower the hydration number of the metal ions was estimated compared to the bulk value. In particular, the dynamics of the metal ions were found to support well the trend, followed by the primary hydration number as well as the order of the maximum adsorption capacity (qmax) exhibited by the different CNTs. The present study provides insights and quantitative information for the adsorption of metal ions with functionalized CNTs, which might be very useful for the exploration of the myriads of nano-adsorbent based experiments and thus future technology.
中文翻译:

量身定制用于吸附重金属离子的碳纳米管:分子动力学和实验研究†
由于人口激增或工业化进程加快而造成的污染水源需要对人类健康产生不利影响,因此需要对其进行处理。CNT在吸附领域中变得非常流行,其在具有各种官能团的官能化作用下可以用作有效的吸附剂。本研究探讨了Cd ++ / Hg ++对羧基(COOH),胺(CONH(CH 2)2 NH 2)和硫(CONH(CH 2)2SH)的CNT,使用分子动力学(MD)模拟。该研究调查了在水性和酸性环境中吸附的分子水平事件。MD研究很好地捕获了实验观察到的碳纳米管对金属离子的Langmuir型吸附等温线。发现酸的存在由于与H 3 O +离子的竞争而降低了金属离子的吸附。结果建立了胺基碳纳米管在硫基碳纳米管上的吸附Cd ++离子的使用。但是,对于汞++的吸附,发现基于胺的CNT和基于硫的CNT都同样合适。模拟轨迹可视化了吸附离子的结合位点,对于大多数官能团,它们优先位于O原子附近,并且在较高浓度下也位于胺的N原子附近。另外,使用吸附相关函数和相应的停留时间分布来确定结合强度。此外,还通过以下方式探索了丰富的结构和动力学事件RDF,CN和扩散系数。与本体值相比,官能化的CNT的吸附能力越高,金属离子的水合数估计越低。特别地,发现金属离子的动力学很好地支持了这种趋势,其次是不同的CNT显示的一次水合数以及最大吸附容量(q max)的顺序。本研究为功能化碳纳米管吸附金属离子提供了见识和定量信息,这可能对探索无数基于纳米吸附剂的实验以及未来的技术非常有用。
更新日期:2018-08-16
中文翻译:

量身定制用于吸附重金属离子的碳纳米管:分子动力学和实验研究†
由于人口激增或工业化进程加快而造成的污染水源需要对人类健康产生不利影响,因此需要对其进行处理。CNT在吸附领域中变得非常流行,其在具有各种官能团的官能化作用下可以用作有效的吸附剂。本研究探讨了Cd ++ / Hg ++对羧基(COOH),胺(CONH(CH 2)2 NH 2)和硫(CONH(CH 2)2SH)的CNT,使用分子动力学(MD)模拟。该研究调查了在水性和酸性环境中吸附的分子水平事件。MD研究很好地捕获了实验观察到的碳纳米管对金属离子的Langmuir型吸附等温线。发现酸的存在由于与H 3 O +离子的竞争而降低了金属离子的吸附。结果建立了胺基碳纳米管在硫基碳纳米管上的吸附Cd ++离子的使用。但是,对于汞++的吸附,发现基于胺的CNT和基于硫的CNT都同样合适。模拟轨迹可视化了吸附离子的结合位点,对于大多数官能团,它们优先位于O原子附近,并且在较高浓度下也位于胺的N原子附近。另外,使用吸附相关函数和相应的停留时间分布来确定结合强度。此外,还通过以下方式探索了丰富的结构和动力学事件RDF,CN和扩散系数。与本体值相比,官能化的CNT的吸附能力越高,金属离子的水合数估计越低。特别地,发现金属离子的动力学很好地支持了这种趋势,其次是不同的CNT显示的一次水合数以及最大吸附容量(q max)的顺序。本研究为功能化碳纳米管吸附金属离子提供了见识和定量信息,这可能对探索无数基于纳米吸附剂的实验以及未来的技术非常有用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号