Joule ( IF 38.6 ) Pub Date : 2018-08-09 , DOI: 10.1016/j.joule.2018.07.019 Xuefeng Wang , Yejing Li , Xuanxuan Bi , Lu Ma , Tianpin Wu , Mahsa Sina , Shen Wang , Minghao Zhang , Judith Alvarado , Bingyu Lu , Abhik Banerjee , Khalil Amine , Jun Lu , Ying Shirley Meng
|
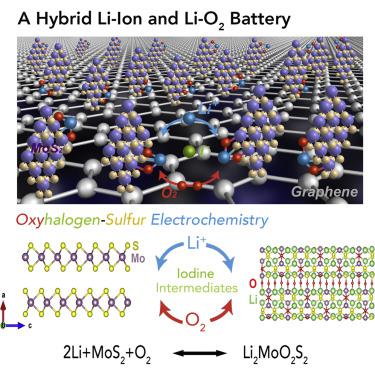
The large voltage hysteresis between charge and discharge results in significant energy loss, which hinders practical application of the high-energy Li-O2 battery. Oxyhalogen-sulfur electrochemistry offers a new hybrid Li-ion/Li-O2 battery, where both Li ions and O anions are reversibly stored in the MoS2 structure. A Li2MoO2S2 compound is formed as the main discharge product that has never been previously observed in the literature. The reaction mechanism and the structure of the Li2MoO2S2 are probed by Raman spectroscopy, X-ray photoelectron spectroscopy, X-ray absorption spectroscopy, differential electrochemical mass spectrometry, and UV-visible spectroscopy. The results show that the MoS2 is oxidized during discharge and is recovered during charge. The iodine intermediates play an important role in triggering the sequence of electrochemical and chemical reactions in the cell. The Li2MoO2S2 is isostructural to the Li2MoO4 rather than adopting structures of other known molybdenum oxysulfides.
中文翻译:

氧-硫-硫化学作用形成的混合锂离子和Li-O 2电池
充电和放电之间的大电压滞后导致明显的能量损失,这阻碍了高能量Li-O 2电池的实际应用。氢氧硫电化学技术提供了一种新的锂离子/ Li-O 2混合电池,其中锂离子和O阴离子可逆地存储在MoS 2结构中。形成Li 2 MoO 2 S 2化合物作为主要放电产物,这在文献中从未见过。Li 2 MoO 2 S 2的反应机理与结构可以通过拉曼光谱,X射线光电子能谱,X射线吸收光谱,差示电化学质谱和UV可见光谱进行探测。结果表明,MoS 2在放电期间被氧化并且在充电期间被回收。碘中间体在触发细胞中电化学和化学反应的顺序中起着重要作用。Li 2 MoO 2 S 2与Li 2 MoO 4是同构的,而不是采用其他已知的氧硫化钼的结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号