当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of K-RAS4B by a Unique Mechanism of Action: Stabilizing Membrane-Dependent Occlusion of the Effector-Binding Site
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-08-16 , DOI: 10.1016/j.chembiol.2018.07.009 Zhenhao Fang 1 , Christopher B Marshall 1 , Tadateru Nishikawa 1 , Alvar D Gossert 2 , Johanna M Jansen 3 , Wolfgang Jahnke 4 , Mitsuhiko Ikura 1
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-08-16 , DOI: 10.1016/j.chembiol.2018.07.009 Zhenhao Fang 1 , Christopher B Marshall 1 , Tadateru Nishikawa 1 , Alvar D Gossert 2 , Johanna M Jansen 3 , Wolfgang Jahnke 4 , Mitsuhiko Ikura 1
Affiliation
|
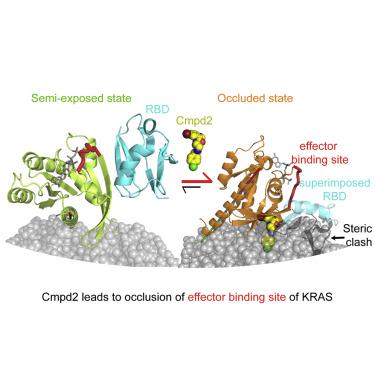
KRASis frequently mutated in several of the most lethal types of cancer; however, the KRAS protein has proven a challenging drug target. K-RAS4B must be localized to the plasma membrane by prenylation to activate oncogenic signaling, thus we endeavored to target the protein-membrane interface with small-molecule compounds. While all reported lead compounds have low affinity for KRAS in solution, the potency of Cmpd2 was strongly enhanced when prenylated K-RAS4B is associated with a lipid bilayer. We have elucidated a unique mechanism of action of Cmpd2, which simultaneously engages a shallow pocket on KRAS and associates with the lipid bilayer, thereby stabilizing KRAS in an orientation in which the membrane occludes its effector-binding site, reducing RAF binding and impairing activation of RAF. Furthermore, enrichment of Cmpd2 on the bilayer enhances potency by promoting interaction with KRAS. This insight reveals a novel approach to developing inhibitors of membrane-associated proteins.
中文翻译:

通过独特的作用机制抑制 K-RAS4B:稳定效应器结合位点的膜依赖性闭塞
KRASis 经常在几种最致命的癌症类型中发生突变;然而,KRAS 蛋白已被证明是一个具有挑战性的药物靶点。K-RAS4B 必须通过异戊二烯化定位于质膜以激活致癌信号,因此我们努力用小分子化合物靶向蛋白质 - 膜界面。尽管所有报道的先导化合物对溶液中的 KRAS 亲和力较低,但当异戊二烯化 K-RAS4B 与脂质双层相关联时,Cmpd2 的效力会大大增强。我们阐明了 Cmpd2 的独特作用机制,它同时与 KRAS 上的浅口袋结合并与脂质双层结合,从而使 KRAS 稳定在膜封闭其效应结合位点的方向,减少 RAF 结合并削弱皇家空军。此外,双层上 Cmpd2 的富集通过促进与 KRAS 的相互作用来增强效力。这一见解揭示了一种开发膜相关蛋白抑制剂的新方法。
更新日期:2018-11-15
中文翻译:

通过独特的作用机制抑制 K-RAS4B:稳定效应器结合位点的膜依赖性闭塞
KRASis 经常在几种最致命的癌症类型中发生突变;然而,KRAS 蛋白已被证明是一个具有挑战性的药物靶点。K-RAS4B 必须通过异戊二烯化定位于质膜以激活致癌信号,因此我们努力用小分子化合物靶向蛋白质 - 膜界面。尽管所有报道的先导化合物对溶液中的 KRAS 亲和力较低,但当异戊二烯化 K-RAS4B 与脂质双层相关联时,Cmpd2 的效力会大大增强。我们阐明了 Cmpd2 的独特作用机制,它同时与 KRAS 上的浅口袋结合并与脂质双层结合,从而使 KRAS 稳定在膜封闭其效应结合位点的方向,减少 RAF 结合并削弱皇家空军。此外,双层上 Cmpd2 的富集通过促进与 KRAS 的相互作用来增强效力。这一见解揭示了一种开发膜相关蛋白抑制剂的新方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号