当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Detecting and Imaging O-GlcNAc Sites Using Glycosyltransferases: A Systematic Approach to Study O-GlcNAc
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-08-09 , DOI: 10.1016/j.chembiol.2018.07.007 Zhengliang L. Wu , Timothy J. Tatge , Alex E. Grill , Yonglong Zou
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-08-09 , DOI: 10.1016/j.chembiol.2018.07.007 Zhengliang L. Wu , Timothy J. Tatge , Alex E. Grill , Yonglong Zou
|
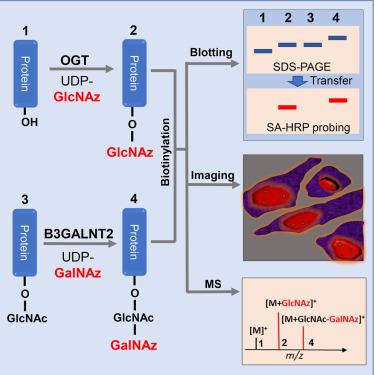
O-GlcNAcylation is a reversible serine/threonine glycosylation for regulating protein activity and availability inside cells. In a given protein,O-GlcNAcylated and unoccupiedO-linked β-N-acetylglucosamine (O-GlcNAc) sites are referred to as closed and open sites, respectively. The balance between open and closed sites is believed to be dynamically regulated. In this report, closed sites are detected usingin vitroincorporation of GalNAz by B3GALNT2, and open sites are detected byin vitroincorporation of GlcNAz byO-GlcNAc transferase (OGT), via click chemistry. For assessing totalO-GlcNAc sites, a sample isO-GlcNAcylatedin vitroby OGT before detecting by B3GALNT2. The methods are demonstrated on purified recombinant proteins including CK2, AKT1, and PFKFB3, and cellular extracts of HEK cells. ThroughO-GlcNAc imaging, the modification degree ofO-GlcNAc in nuclei of Chinese hamster ovary cells was estimated. The detection and imaging of both open and closedO-GlcNAc sites provide a systematic approach to study this important post-translational modification.
更新日期:2018-11-15











































 京公网安备 11010802027423号
京公网安备 11010802027423号