Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2018-08-16 , DOI: 10.1016/j.jhazmat.2018.08.039 Yufeng Zhao , Jong-Won Choi , John Kwame Bediako , Myung-Hee Song , Shuo Lin , Chul-Woong Cho , Yeoung-Sang Yun
|
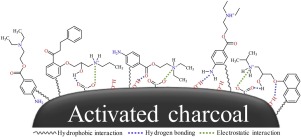
Due to high mobility and specific toxic actions of the ionizable pharmaceuticals in surface water with a normal range of pH, the pharmaceuticals should be removed before being discharged. Therefore, this study investigated the adsorptive interactions between cationic pharmaceuticals and a popular adsorbent (i.e., activated charcoal) frequently used in water treatment processes. For that, we performed isotherm experiments and then the results were plotted by Langmuir model to determine the adsorption affinity (b) and capacity (qm). Afterwards, to interpret the adsorption behaviors, two simple prediction models were developed based on quantitative structure-activity relationships (QSAR). In the modelling, molecular weight (MW), polar surface area (PSA), and octanol-water partitioning coefficient (log P) were used as model parameters. In the results, the combinations of these three parameters could predict the adsorption affinity and capacity in R2 of 0.85 and 0.80, respectively. The robustness of models was validated by leave-one-out cross-validation (Q2LOO) and the estimated Q2LOO values were 0.60 and 0.55 for the adsorption affinity and capacity, respectively, which are higher than the acceptability of standard i.e., 0.5.
中文翻译:

阳离子药物对活性炭的吸附相互作用:实验测定和QSAR建模
由于可电离的药物在具有正常pH值的地表水中具有较高的迁移率和特定的毒性作用,因此在排放前应将其清除。因此,本研究调查了阳离子药物与水处理过程中经常使用的流行吸附剂(即活性炭)之间的吸附相互作用。为此,我们进行了等温线实验,然后通过Langmuir模型绘制结果,以确定吸附亲和力(b)和容量(q m)。然后,为了解释吸附行为,基于定量构效关系(QSAR)开发了两个简单的预测模型。在建模中,分子量(MW),极性表面积(PSA)和辛醇-水分配系数(log P)用作模型参数。结果,这三个参数的组合可以分别预测吸附亲和力和在R 2中的容量分别为0.85和0.80。模型的稳健性通过留一法交叉验证(Q 2 LOO)和估计的Q 2 LOO进行了验证 吸附亲和力和吸附力分别为0.60和0.55,高于标准品的可接受性0.5。











































 京公网安备 11010802027423号
京公网安备 11010802027423号