当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Progression of Self-Assembly of Amelogenin Protein Supramolecular Structures in Simulated Enamel Fluid
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-08-16 00:00:00 , DOI: 10.1021/acs.biomac.8b00808 Sarah A. Engelberth 1 , Margot S. Bacino 1 , Shaiba Sandhu 1 , Wu Li 1 , Johan Bonde 2 , Stefan Habelitz 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-08-16 00:00:00 , DOI: 10.1021/acs.biomac.8b00808 Sarah A. Engelberth 1 , Margot S. Bacino 1 , Shaiba Sandhu 1 , Wu Li 1 , Johan Bonde 2 , Stefan Habelitz 1
Affiliation
|
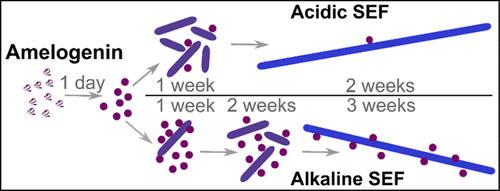
Mechanisms of protein-guided mineralization in enamel, leading to organized fibrillar apatite nanocrystals, remain elusive. In vitro studies reveal recombinant human amelogenin (rH174), a matrix protein templating this process, self-assembles into a variety of structures. This study endeavors to clarify the self-assembly of rH174 in physiologically relevant conditions. Self-assembly in simulated enamel fluid was monitored up to 2 months. At alkali (7.3–8.7) and acidic (5.5–6.1) pH ranges, a distinct progression in formation was observed from nanospheres (17–23 nm) to intermediate-length nanorods, concluding with the formation of long 17–18 nm wide nanoribbons decorated with nanospheres. Assembly in acidic condition progressed quicker to nanoribbons with fewer persistent nanospheres. X-ray diffraction exhibited reflections characteristic of antiparallel β-sheets (4.7 and 9.65 Å), supporting the model of amyloid-like nanoribbon formation. This is the first observation of rH174 nanoribbons at alkaline pH as well as concurrent nanosphere formation, indicating both supramolecular structures are stable together under physiological conditions.
中文翻译:

模拟釉质液中釉蛋白原超分子结构的自组装进展
牙釉质中蛋白质引导的矿化机制(导致有组织的原纤维磷灰石纳米晶体)的机理仍然难以捉摸。体外研究显示重组人牙釉蛋白(rH174)是一种可模拟此过程的基质蛋白,可自组装成多种结构。这项研究致力于阐明生理相关条件下rH174的自组装。在模拟搪瓷液中的自组装进行了长达2个月的监控。在碱性(7.3–8.7)和酸性(5.5–6.1)pH范围内,观察到从纳米球(17–23 nm)到中等长度的纳米棒的形成过程明显不同,最后形成了长17–18 nm宽的纳米带。用纳米球装饰。在酸性条件下的组装进展更快,纳米碳带的持久性纳米球更少。X射线衍射显示反平行β片(4.7和9.65Å)的反射特征,支持淀粉样样纳米带形成模型。这是rH174纳米带在碱性pH下以及同时形成纳米球的首次观察,表明这两种超分子结构在生理条件下都稳定在一起。
更新日期:2018-08-16
中文翻译:

模拟釉质液中釉蛋白原超分子结构的自组装进展
牙釉质中蛋白质引导的矿化机制(导致有组织的原纤维磷灰石纳米晶体)的机理仍然难以捉摸。体外研究显示重组人牙釉蛋白(rH174)是一种可模拟此过程的基质蛋白,可自组装成多种结构。这项研究致力于阐明生理相关条件下rH174的自组装。在模拟搪瓷液中的自组装进行了长达2个月的监控。在碱性(7.3–8.7)和酸性(5.5–6.1)pH范围内,观察到从纳米球(17–23 nm)到中等长度的纳米棒的形成过程明显不同,最后形成了长17–18 nm宽的纳米带。用纳米球装饰。在酸性条件下的组装进展更快,纳米碳带的持久性纳米球更少。X射线衍射显示反平行β片(4.7和9.65Å)的反射特征,支持淀粉样样纳米带形成模型。这是rH174纳米带在碱性pH下以及同时形成纳米球的首次观察,表明这两种超分子结构在生理条件下都稳定在一起。











































 京公网安备 11010802027423号
京公网安备 11010802027423号