当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lactobionic/Folate Dual-Targeted Amphiphilic Maltodextrin-Based Micelles for Targeted Codelivery of Sulfasalazine and Resveratrol to Hepatocellular Carcinoma
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-08-15 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00428 Doaa M. Anwar , Sherine N. Khattab 1 , Maged W. Helmy 2 , Mohamed K. Kamal 3 , Adnan A. Bekhit 4 , Kadria A. Elkhodairy , Ahmed O. Elzoghby 5, 6
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-08-15 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00428 Doaa M. Anwar , Sherine N. Khattab 1 , Maged W. Helmy 2 , Mohamed K. Kamal 3 , Adnan A. Bekhit 4 , Kadria A. Elkhodairy , Ahmed O. Elzoghby 5, 6
Affiliation
|
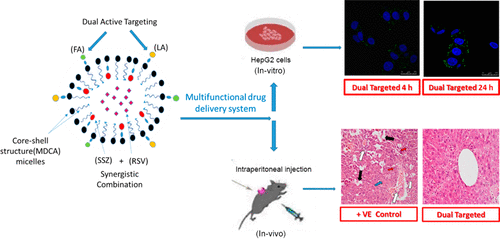
In this study, promising approaches of dual-targeted micelles and drug–polymer conjugation were combined to enable injection of poorly soluble anticancer drugs together with site-specific drug release. Ursodeoxycholic acid (UDCA) as a hepatoprotective agent was grafted to maltodextrin (MD) via carbodiimide coupling to develop amphiphilic maltodextrin-ursodeoxycholic acid (MDCA)-based micelles. Sulfasalazine (SSZ), as a novel anticancer agent, was conjugated via a tumor-cleavable ester bond to MD backbone to obtain tumor-specific release, whereas resveratrol (RSV) was physically entrapped within the hydrophobic micellar core. For maximal tumor-targeting, both folic acid (FA) and lactobionic acid (LA) were coupled to the surface of micelles to obtain dual-targeted micelles. The decrease of critical micelle concentration (CMC) from 0.012 to 0.006 mg/mL declares the significance of a dual hydrophobicized core of micelles by both UDCA and SSZ. The dual-targeted micelles showed a great hemocompatibility, as well as enhanced cytotoxicity and internalization into HepG-2 liver cancer cells via binding to overexpressed folate and asialoglycoprotein receptors. In vivo, the micelles demonstrated superior antitumor effects revealed as reduction in the liver/body weight ratio, inhibition of angiogenesis, and enhanced apoptosis. Overall, combined strategies of dual active targeted micelles with bioresponsive drug conjugation could be utilized as a promising approach for tumor-targeted drug delivery.
中文翻译:

乳酸/叶酸双目标两亲麦芽糖糊精为基础的胶束的磺胺嘧啶和白藜芦醇靶向肝癌的代码传递。
在这项研究中,结合了双重靶向胶束和药物-聚合物结合的有前途的方法,可以注射难溶性抗癌药物并释放特定部位的药物。通过碳二亚胺偶联将熊去氧胆酸(UDCA)作为保肝剂移植到麦芽糖糊精(MD)上,从而开发出基于两亲性麦芽糖糊精-熊去氧胆酸(MDCA)的胶束。柳氮磺吡啶(SSZ)作为一种新型抗癌药,通过肿瘤可裂解的酯键与MD骨架结合,从而获得了肿瘤特异性释放,而白藜芦醇(RSV)则被物理地包裹在疏水胶束核心中。为了最大程度地靶向肿瘤,将叶酸(FA)和乳糖酸(LA)均与胶束表面偶联以获得双靶向胶束。临界胶束浓度(CMC)从0.012降低到0。006 mg / mL通过UDCA和SSZ声明了胶束双重疏水核心的重要性。通过与过表达的叶酸和去唾液酸糖蛋白受体结合,双重靶向的胶束具有很好的血液相容性,并增强了细胞毒性和内化作用进入HepG-2肝癌细胞。在体内,该胶束表现出优异的抗肿瘤作用,表现为肝脏/体重比的降低,血管生成的抑制和细胞凋亡的增强。总体而言,双重活性靶向胶束与生物反应性药物结合的联合策略可以用作有针对性的肿瘤靶向药物递送方法。以及通过与过度表达的叶酸和去唾液酸糖蛋白受体结合而增强的细胞毒性和内化作用进入HepG-2肝癌细胞。在体内,该胶束表现出优异的抗肿瘤作用,表现为肝脏/体重比的降低,血管生成的抑制和细胞凋亡的增强。总体而言,双重活性靶向胶束与生物反应性药物结合的联合策略可以用作有针对性的肿瘤靶向药物递送方法。以及通过与过度表达的叶酸和去唾液酸糖蛋白受体结合而增强的细胞毒性和内化作用进入HepG-2肝癌细胞。在体内,该胶束表现出优异的抗肿瘤作用,表现为肝脏/体重比的降低,血管生成的抑制和细胞凋亡的增强。总体而言,双重活性靶向胶束与生物反应性药物结合的联合策略可以用作有针对性的肿瘤靶向药物递送方法。
更新日期:2018-08-15
中文翻译:

乳酸/叶酸双目标两亲麦芽糖糊精为基础的胶束的磺胺嘧啶和白藜芦醇靶向肝癌的代码传递。
在这项研究中,结合了双重靶向胶束和药物-聚合物结合的有前途的方法,可以注射难溶性抗癌药物并释放特定部位的药物。通过碳二亚胺偶联将熊去氧胆酸(UDCA)作为保肝剂移植到麦芽糖糊精(MD)上,从而开发出基于两亲性麦芽糖糊精-熊去氧胆酸(MDCA)的胶束。柳氮磺吡啶(SSZ)作为一种新型抗癌药,通过肿瘤可裂解的酯键与MD骨架结合,从而获得了肿瘤特异性释放,而白藜芦醇(RSV)则被物理地包裹在疏水胶束核心中。为了最大程度地靶向肿瘤,将叶酸(FA)和乳糖酸(LA)均与胶束表面偶联以获得双靶向胶束。临界胶束浓度(CMC)从0.012降低到0。006 mg / mL通过UDCA和SSZ声明了胶束双重疏水核心的重要性。通过与过表达的叶酸和去唾液酸糖蛋白受体结合,双重靶向的胶束具有很好的血液相容性,并增强了细胞毒性和内化作用进入HepG-2肝癌细胞。在体内,该胶束表现出优异的抗肿瘤作用,表现为肝脏/体重比的降低,血管生成的抑制和细胞凋亡的增强。总体而言,双重活性靶向胶束与生物反应性药物结合的联合策略可以用作有针对性的肿瘤靶向药物递送方法。以及通过与过度表达的叶酸和去唾液酸糖蛋白受体结合而增强的细胞毒性和内化作用进入HepG-2肝癌细胞。在体内,该胶束表现出优异的抗肿瘤作用,表现为肝脏/体重比的降低,血管生成的抑制和细胞凋亡的增强。总体而言,双重活性靶向胶束与生物反应性药物结合的联合策略可以用作有针对性的肿瘤靶向药物递送方法。以及通过与过度表达的叶酸和去唾液酸糖蛋白受体结合而增强的细胞毒性和内化作用进入HepG-2肝癌细胞。在体内,该胶束表现出优异的抗肿瘤作用,表现为肝脏/体重比的降低,血管生成的抑制和细胞凋亡的增强。总体而言,双重活性靶向胶束与生物反应性药物结合的联合策略可以用作有针对性的肿瘤靶向药物递送方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号