当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Design of Novel EphA2 Agonistic Agents with Nanomolar Affinity in Vitro and in Cell
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-15 00:00:00 , DOI: 10.1021/acschembio.8b00556 Luca Gambini 1 , Ahmed F. Salem 1 , Parima Udompholkul 1 , Xiao-Feng Tan 2 , Carlo Baggio 1 , Neh Shah 1 , Alexander Aronson 1 , Jikui Song 2 , Maurizio Pellecchia 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-15 00:00:00 , DOI: 10.1021/acschembio.8b00556 Luca Gambini 1 , Ahmed F. Salem 1 , Parima Udompholkul 1 , Xiao-Feng Tan 2 , Carlo Baggio 1 , Neh Shah 1 , Alexander Aronson 1 , Jikui Song 2 , Maurizio Pellecchia 1
Affiliation
|
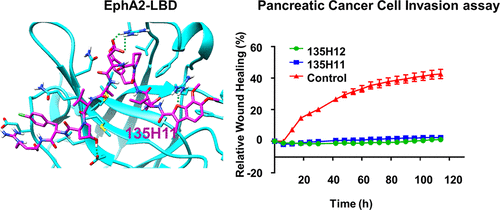
EphA2 overexpression is invariably associated with poor prognosis and development of aggressive metastatic cancers in pancreatic, prostate, lung, ovarian, and breast cancers and melanoma. Recent efforts from our laboratories identified a number of agonistic peptides targeting the ligand-binding domain of the EphA2 receptor. The individual agents, however, were still relatively weak in affinities (micromolar range) that precluded detailed structural studies on the mode of action. Using a systematic optimization of the 12-mer peptide mimetic 123B9, we were able to first derive an agent that displayed a submicromolar affinity for the receptor. This agent enabled cocrystallization with the EphA2 ligand-binding domain providing for the first time the structural basis for their agonistic mechanism of action. In addition, the atomic coordinates of the complex enabled rapid iterations of structure-based optimizations that resulted in a novel agonistic agent, named 135H11, with a nanomolar affinity for the receptor, as demonstrated by in vitro binding assays (isothermal titration calorimetry measurements), and a biochemical displacement assay. As we have recently demonstrated, the cellular activity of these agents is further increased by synthesizing dimeric versions of the compounds. Hence, we report that a dimeric version of 135H11 is extremely effective at low nanomolar concentrations to induce cellular receptor activation, internalization, and inhibition of cell migration in a pancreatic cancer cell line. Given the pivotal role of EphA2 in tumor growth, angiogenesis, drug resistance, and metastasis, these agents, and the associated structural studies, provide significant advancements in the field for the development of novel EphA2-targeting therapeutics or diagnostics.
中文翻译:

体外和细胞内具有纳摩尔亲和力的新型EphA2激动剂的基于结构的设计
EphA2过表达总是与胰腺癌,前列腺癌,肺癌,卵巢癌,乳腺癌和黑色素瘤中侵袭性转移癌的不良预后和发展有关。我们实验室的最新成果确定了许多靶向EphA2受体配体结合结构域的激动肽。但是,单个试剂的亲和力(微摩尔范围)仍然相对较弱,因此无法对作用方式进行详细的结构研究。使用12-mer肽模拟物123B9的系统优化,我们能够首先衍生出对受体表现出亚微摩尔亲和力的药物。该试剂能够与EphA2配体结合结构域共结晶,这首次为其激动作用机理提供了结构基础。此外,体外结合测定(等温滴定量热法测量)和生化置换测定。正如我们最近所证明的,这些试剂的细胞活性通过合成化合物的二聚体形式而进一步提高。因此,我们报道135H11的二聚体版本在低纳摩尔浓度下极有效地诱导胰腺癌细胞株中的细胞受体活化,内化和细胞迁移的抑制。鉴于EphA2在肿瘤生长,血管生成,耐药性和转移中的关键作用,这些药物以及相关的结构研究为开发靶向EphA2的新型治疗剂或诊断剂提供了重要的进展。
更新日期:2018-08-15
中文翻译:

体外和细胞内具有纳摩尔亲和力的新型EphA2激动剂的基于结构的设计
EphA2过表达总是与胰腺癌,前列腺癌,肺癌,卵巢癌,乳腺癌和黑色素瘤中侵袭性转移癌的不良预后和发展有关。我们实验室的最新成果确定了许多靶向EphA2受体配体结合结构域的激动肽。但是,单个试剂的亲和力(微摩尔范围)仍然相对较弱,因此无法对作用方式进行详细的结构研究。使用12-mer肽模拟物123B9的系统优化,我们能够首先衍生出对受体表现出亚微摩尔亲和力的药物。该试剂能够与EphA2配体结合结构域共结晶,这首次为其激动作用机理提供了结构基础。此外,体外结合测定(等温滴定量热法测量)和生化置换测定。正如我们最近所证明的,这些试剂的细胞活性通过合成化合物的二聚体形式而进一步提高。因此,我们报道135H11的二聚体版本在低纳摩尔浓度下极有效地诱导胰腺癌细胞株中的细胞受体活化,内化和细胞迁移的抑制。鉴于EphA2在肿瘤生长,血管生成,耐药性和转移中的关键作用,这些药物以及相关的结构研究为开发靶向EphA2的新型治疗剂或诊断剂提供了重要的进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号