当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper (II)/RuPHOX‐Catalyzed Enantioselective Mannich‐Type Reaction of Glycine Schiff Bases with Cyclic Ketimines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-30 , DOI: 10.1002/adsc.201800850 Qihang Shao 1 , Liang Wu 1 , Jianzhong Chen 1 , Ilya D. Gridnev 2 , Guoqiang Yang 1 , Fang Xie 1 , Wanbin Zhang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-30 , DOI: 10.1002/adsc.201800850 Qihang Shao 1 , Liang Wu 1 , Jianzhong Chen 1 , Ilya D. Gridnev 2 , Guoqiang Yang 1 , Fang Xie 1 , Wanbin Zhang 1
Affiliation
|
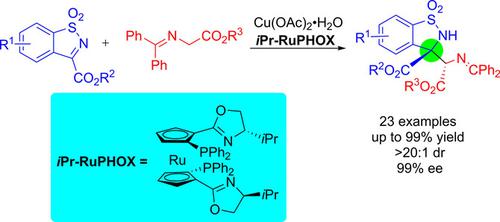
A Cu(II)/RuPHOX‐catalyzed enantioselective Mannich‐type reaction of glycine Schiff bases with cyclic ketimines was developed, affording chiral α,ß‐diamino acid derivatives in good yields with moderate to good ee and dr values. This provides an efficient methodology for furnishing chiral Cβ‐tetrasubstituted α,β‐diamino acid precursors. The catalytic system is compatible with a series of substrates. In addition, an interesting nonlinear effect of the catalyst's enantiomeric composition on reaction enantioselectivity was observed.
中文翻译:

铜(II)/ RuPHOX催化甘氨酸席夫碱与环酮亚胺的对映选择性曼尼希型反应
开发了Cu(II)/ RuPHOX催化的甘氨酸席夫碱与环状酮亚胺的对映选择性曼尼希型反应,可提供高收率的手性α,β-二氨基酸衍生物,且ee和dr值适中。这提供了用于供给手性℃的高效方法β -tetrasubstituted α,β -二氨基酸前体。该催化系统与一系列底物兼容。另外,观察到催化剂的对映体组成对反应对映体选择性的有趣的非线性影响。
更新日期:2018-10-30
中文翻译:

铜(II)/ RuPHOX催化甘氨酸席夫碱与环酮亚胺的对映选择性曼尼希型反应
开发了Cu(II)/ RuPHOX催化的甘氨酸席夫碱与环状酮亚胺的对映选择性曼尼希型反应,可提供高收率的手性α,β-二氨基酸衍生物,且ee和dr值适中。这提供了用于供给手性℃的高效方法β -tetrasubstituted α,β -二氨基酸前体。该催化系统与一系列底物兼容。另外,观察到催化剂的对映体组成对反应对映体选择性的有趣的非线性影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号