当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Atropisomeric Factors Governing the Selectivity of Pyrimido-benzodiazipinones as Inhibitors of Kinases and Bromodomains.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-31 , DOI: 10.1021/acschembio.7b00638 Jinhua Wang 1, 2 , Tatiana Erazo 3 , Fleur M Ferguson 1, 2 , Dennis L Buckley 1 , Nestor Gomez 3 , Pau Muñoz-Guardiola 3 , Nora Diéguez-Martínez 3 , Xianming Deng 1, 2 , Mingfeng Hao 1 , Walter Massefski 1 , Oleg Fedorov 4 , Nana Kwaku Offei-Addo 1 , Paul M Park 1 , Lingling Dai 1 , Amy DiBona 5 , Kelly Becht 5 , Nam Doo Kim 6 , Michael R McKeown 7 , Justin M Roberts 7 , Jinwei Zhang 8 , Taebo Sim 9, 10 , Dario R Alessi 8 , James E Bradner 7, 11 , Jose M Lizcano 3 , Stephen C Blacklow 1, 2 , Jun Qi 1, 11 , Xiang Xu 1, 2 , Nathanael S Gray 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-31 , DOI: 10.1021/acschembio.7b00638 Jinhua Wang 1, 2 , Tatiana Erazo 3 , Fleur M Ferguson 1, 2 , Dennis L Buckley 1 , Nestor Gomez 3 , Pau Muñoz-Guardiola 3 , Nora Diéguez-Martínez 3 , Xianming Deng 1, 2 , Mingfeng Hao 1 , Walter Massefski 1 , Oleg Fedorov 4 , Nana Kwaku Offei-Addo 1 , Paul M Park 1 , Lingling Dai 1 , Amy DiBona 5 , Kelly Becht 5 , Nam Doo Kim 6 , Michael R McKeown 7 , Justin M Roberts 7 , Jinwei Zhang 8 , Taebo Sim 9, 10 , Dario R Alessi 8 , James E Bradner 7, 11 , Jose M Lizcano 3 , Stephen C Blacklow 1, 2 , Jun Qi 1, 11 , Xiang Xu 1, 2 , Nathanael S Gray 1, 2
Affiliation
|
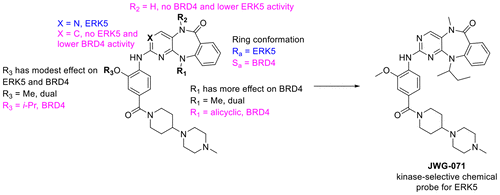
Bromodomains have been pursued intensively over the past several years as emerging targets for the development of anticancer and anti-inflammatory agents. It has recently been shown that some kinase inhibitors are able to potently inhibit the bromodomains of BRD4. The clinical activities of PLK inhibitor BI-2536 and JAK2-FLT3 inhibitor TG101348 have been attributed to this unexpected polypharmacology, indicating that dual-kinase/bromodomain activity may be advantageous in a therapeutic context. However, for target validation and biological investigation, a more selective target profile is desired. Here, we report that benzo[e]pyrimido-[5,4- b]diazepine-6(11H)-ones, versatile ATP-site directed kinase pharmacophores utilized in the development of inhibitors of multiple kinases, including several previously reported kinase chemical probes, are also capable of exhibiting potent BRD4-dependent pharmacology. Using a dual kinase-bromodomain inhibitor of the kinase domains of ERK5 and LRRK2, and the bromodomain of BRD4 as a case study, we define the structure-activity relationships required to achieve dual kinase/BRD4 activity, as well as how to direct selectivity toward inhibition of either ERK5 or BRD4. This effort resulted in identification of one of the first reported kinase-selective chemical probes for ERK5 (JWG-071), a BET selective inhibitor with 1 μM BRD4 IC50 (JWG-115), and additional inhibitors with rationally designed polypharmacology (JWG-047, JWG-069). Co-crystallography of seven representative inhibitors with the first bromodomain of BRD4 demonstrate that distinct atropisomeric conformers recognize the kinase ATP-site and the BRD4 acetyl lysine binding site, conformational preferences supported by rigid docking studies.
中文翻译:

结构和阻转异构因素控制嘧啶-苯并二氮杂二酮作为激酶和溴结构域抑制剂的选择性。
在过去的几年中,Bromodomains被广泛地追求作为抗癌和抗炎剂开发的新兴目标。最近显示出一些激酶抑制剂能够有效地抑制BRD4的溴结构域。PLK抑制剂BI-2536和JAK2-FLT3抑制剂TG101348的临床活性已归因于这种出乎意料的多元药理学,这表明双重激酶/溴结构域活性在治疗方面可能是有利的。但是,对于靶标验证和生物学研究,需要更具选择性的靶标谱。在这里,我们报告说,苯并[e]嘧啶基-[5,4-b]二氮杂-6(11H)-ones是一种多功能ATP定位的激酶药效团,可用于开发多种激酶的抑制剂,包括几种先前报道的激酶化学物质探针 也具有有效的BRD4依赖性药理作用。以ERK5和LRRK2激酶结构域的双激酶-溴结构域抑制剂以及BRD4的溴结构域为案例研究,我们定义了实现双重激酶/ BRD4活性所需的结构-活性关系,以及如何将选择性引导至抑制ERK5或BRD4。这项工作导致鉴定出了最早报道的ERK5激酶选择性化学探针之一(JWG-071),具有1μMBRD4 IC50的BET选择性抑制剂(JWG-115)和具有合理设计的多药理学的其他抑制剂(JWG-047) ,JWG-069)。七个具有代表性的抑制剂与BRD4的第一个溴结构域的共结晶表明,不同的阻转异构体构象异构体识别激酶ATP位点和BRD4乙酰赖氨酸结合位点,
更新日期:2018-08-13
中文翻译:

结构和阻转异构因素控制嘧啶-苯并二氮杂二酮作为激酶和溴结构域抑制剂的选择性。
在过去的几年中,Bromodomains被广泛地追求作为抗癌和抗炎剂开发的新兴目标。最近显示出一些激酶抑制剂能够有效地抑制BRD4的溴结构域。PLK抑制剂BI-2536和JAK2-FLT3抑制剂TG101348的临床活性已归因于这种出乎意料的多元药理学,这表明双重激酶/溴结构域活性在治疗方面可能是有利的。但是,对于靶标验证和生物学研究,需要更具选择性的靶标谱。在这里,我们报告说,苯并[e]嘧啶基-[5,4-b]二氮杂-6(11H)-ones是一种多功能ATP定位的激酶药效团,可用于开发多种激酶的抑制剂,包括几种先前报道的激酶化学物质探针 也具有有效的BRD4依赖性药理作用。以ERK5和LRRK2激酶结构域的双激酶-溴结构域抑制剂以及BRD4的溴结构域为案例研究,我们定义了实现双重激酶/ BRD4活性所需的结构-活性关系,以及如何将选择性引导至抑制ERK5或BRD4。这项工作导致鉴定出了最早报道的ERK5激酶选择性化学探针之一(JWG-071),具有1μMBRD4 IC50的BET选择性抑制剂(JWG-115)和具有合理设计的多药理学的其他抑制剂(JWG-047) ,JWG-069)。七个具有代表性的抑制剂与BRD4的第一个溴结构域的共结晶表明,不同的阻转异构体构象异构体识别激酶ATP位点和BRD4乙酰赖氨酸结合位点,











































 京公网安备 11010802027423号
京公网安备 11010802027423号