Talanta ( IF 5.6 ) Pub Date : 2018-08-13 , DOI: 10.1016/j.talanta.2018.08.034 Guoliang Liu , Da-Qian Feng , Zhiyi Li , Yang Feng
|
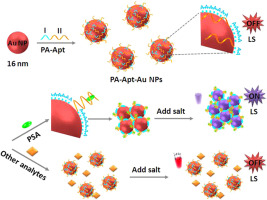
An ultrasensitive and selective light-scattering (LS) aptasensor for prostate specific antigen (PSA) was developed based on bifunctional DNA decorated gold nanoparticles (Au NPs) by use of target stimuli-responsive assembly. Both binding sequence poly adenine (poly(dA), Domain I) and the recognition sequence aptamer (Domain II) of bifunctional ssDNA were in favor of the sensitivity for the fabricated light-scattering aptasensor due to modulated the lateral spacing of DNA on Au NPs surface and possessed strong antigen binding affinity, respectively. And they efficiently promoted the Au NPs aggregation and activated the signal of light-scattering of aptasensor. The formation of aptamer-PSA complex induced conformational transformation of the aptamer on the surface of the Au NPs-based aptasensor and then tuned the interparticle distance of gold nanoparticle assemblies. The larger scattering particles, the higher light-scattering intensity. Target stimuli-responsive aggregation behavior of Au NPs lit up the light-scattering signal of aptasensor. As the concentration of PSA increased, the light-scattering intensity gradually enhanced. The aptasensor response for PSA detection was in the linear range from 0.01 ng/mL to 20 ng/mL with the detection limit of 2 pg/mL. The thiol-free Au NPs-based light-scattering aptasensor has exceptional performances with ultrasensitivity and high selectivity besides the advantage of economic and facile fabrication. Moreover, the proposed target-activated aptasensor was applied for the detection of PSA in serum samples and demonstrated great potential in clinical applications.
中文翻译:

基于靶标可激活的金纳米粒子使用刺激反应性聚集对蛋白质生物标志物的适体性
基于双功能DNA修饰的金纳米颗粒(Au NPs),通过使用靶标刺激响应组件,开发了一种用于前列腺特异性抗原(PSA)的超灵敏和选择性光散射(LS)适体传感器。双功能ssDNA的结合序列聚腺嘌呤(poly(dA),结构域I)和识别序列适体(域II)均对调制的光散射适体传感器具有敏感性,这是由于在Au NP上调节了DNA的侧向间隔表面和具有较强的抗原结合亲和力。并且它们有效地促进了金纳米颗粒的聚集并激活了适体传感器的光散射信号。适体-PSA复合物的形成引起适体在基于Au NPs的适体传感器表面上的构象转变,然后调节金纳米粒子组件的粒子间距离。散射粒子越大,光散射强度越高。Au NPs的目标刺激响应聚集行为照亮了aptasensor的光散射信号。随着PSA浓度的增加,光散射强度逐渐增强。PSA检测的适体传感器响应在0.01 ng / mL到20 ng / mL的线性范围内,检测极限为2 pg / mL。基于硫醇的Au NPs无光散射适体传感器除具有经济且易于制造的优点外,还具有超高灵敏度和高选择性的出色性能。而且,










































 京公网安备 11010802027423号
京公网安备 11010802027423号