Electrochemistry Communications ( IF 4.7 ) Pub Date : 2018-08-14 , DOI: 10.1016/j.elecom.2018.08.006 Jiaxiang Liang , Yun Zhang , Chencheng Song , Diyong Tang , Jie Sun
|
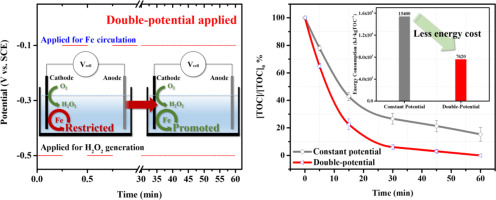
In general, the electro-Fenton degradation potential is set according to the optimal H2O2 generation potential without considering Fe2+/Fe3+ electrocatalytic recycling, resulting in high-energy consumption and iron sludge. In this work, a novel double-potential strategy was provided by alternately applying the optimal H2O2 generation potential and Fe2+/Fe3+ recycling potential during the degradation process. The double-potential method showed a coordination of in-situ H2O2 generation by oxygen reduction reaction (ORR) and Fe2+/Fe3+ recycling. EPR detection indicated that more hydroxyl radicals were produced during the double-potential process compared with the one achieved by constant potential. An outstanding DMP mineralization rate of 94.0% was achieved within 30 min and corresponding energy consumption was 0.762 × 104 kJ kg TOC−1 while the constant potential method reached 73.4% at −0.5 V and corresponding energy consumption was 1.54 × 104 kJ kg TOC−1.
中文翻译:

双电位电Fenton:氧还原反应与Fe 2+ / Fe 3+循环结合的新策略
通常,在不考虑Fe 2+ / Fe 3+电催化循环的情况下,根据最佳的H 2 O 2产生电势来设置Fenton电降解电势,导致高能耗和铁污泥。在这项工作中,通过在降解过程中交替应用最佳H 2 O 2生成电势和Fe 2+ / Fe 3+循环电势,提供了一种新颖的双电势策略。双电位法显示通过氧还原反应(ORR)和Fe 2+ / Fe原位生成H 2 O 23+回收。EPR检测表明,与通过恒定电势获得的羟基自由基相比,在双电势过程中产生了更多的羟基自由基。30分钟内达到了出色的DMP矿化率94.0%,相应的能耗为0.762×10 4 kJ kg TOC -1,而恒电位法在-0.5 V时达到73.4%,相应的能耗为1.54×10 4 kJ kg TOC -1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号