Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-08-12 , DOI: 10.1016/j.bioorg.2018.08.013 Alaa A. Abd Elhameed , Nadia S. El-Gohary , Eman R. El-Bendary , Mona I. Shaaban , Said M. Bayomi
|
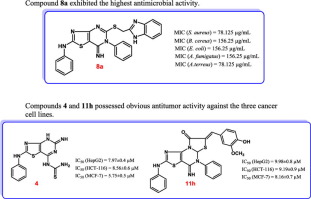
New thiazolopyrimidine and dithiazolopyrimidinone derivatives 2–11 were synthesized and estimated for antimicrobial activity against S. aureus, B. cereus, E. coli, C. albicans, A. fumigatus and A. terreus. The attained results proved that 4, 8a and 11g have significant effectiveness against S. aureus and B. cereus. On the other hand, 7, 10b, 10c and 11h exhibited prominent activity against B. cereus, whereas 8a, 10b and 11g were proved to be active against E. coli. From another point of view, 4 and 8a exhibited promising efficacy against A. fumigatus and A. terreus; moreover, 8a showed outstanding efficacy against C. albicans. Quorum-sensing inhibitory activity of the new compounds was esteemed against C. violaceum, where 7, 8a, 9b, 10a-c, 11d and 11g have acceptable efficacy. In vitro antitumor efficacy of the same compounds against HepG2, HCT-116 and MCF-7 cancer cell lines was also tested. Compounds 4 and 11h showed enhanced effectiveness against the three cell lines, whereas 10b displayed eminent activity against HCT-116 and MCF-7 cells. Moreover, 11a was found to have outstanding activity against MCF-7 cells, while 11i showed promising efficacy against HepG2 cells. The in vitro active antitumor compounds were evaluated for in vivo antitumor effectiveness against EAC in mice, as well as in vitro cytotoxicity against WI38 and WISH normal cells. Results manifested that 4 has the strongest in vivo activity, and that all investigated analogs are less cytotoxic than 5-FU against both normal cell lines. DNA-binding affinity of the active compounds was examined, where 4, 8a, 10c, 11d and 11g,h displayed strong affinity. In silico studies proved that majority of the analyzed compounds are in conformity with the optimum needs for good oral absorption.
中文翻译:

新型噻唑并[4,5- d ]嘧啶和二噻唑并[3,2- a:5',4'- e ]嘧啶酮衍生物的合成及生物学筛选
新噻唑并嘧啶和dithiazolopyrimidinone衍生物2 - 11合成和估计针对抗微生物活性的金黄色葡萄球菌,蜡样芽胞杆菌,大肠杆菌,白色念珠菌,烟曲霉和土曲霉。所达到的结果证明,4,图8A和11g的具有针对显著有效性金黄色葡萄球菌和蜡状芽孢杆菌。在另一方面,7,10B,10C和11H表现出显着的活性对蜡状芽孢杆菌,而8a,10b和11g被证明对大肠杆菌具有活性。从另一个角度来看,4和8a对烟曲霉和土曲霉显示出有希望的功效。此外,8a对白色念珠菌显示出优异的功效。群体感应的新化合物的抑制活性被推崇针对C.堇色,其中7,图8A,图9B,图10A-C ,11D和11克有可接受的功效。还测试了相同化合物对HepG2,HCT-116和MCF-7癌细胞的体外抗肿瘤功效。化合物4和11h对三种细胞系显示出增强的效力,而化合物10b对HCT-116和MCF-7细胞显示出显着活性。此外,发现11a对MCF-7细胞具有杰出的活性,而11i对HepG2细胞显示出有希望的功效。的体外活性的抗肿瘤化合物用于评价体内在小鼠的抗肿瘤效果对EAC,以及在体外对WI38和WISH正常细胞具有细胞毒性。结果表明,4具有最强的体内活性,并且所有研究的类似物对两种正常细胞系的细胞毒性均低于5-FU。DNA结合的活性化合物的亲合性进行了检查,其中4,图8a,图10C,图11D和11克,ħ显示强亲和力。在计算机研究中,证明大多数分析化合物符合良好的口服吸收的最佳需求。











































 京公网安备 11010802027423号
京公网安备 11010802027423号