当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Pauli Principle: Effects on the Wave Function Seen through the Lens of Orbital Overlap
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-08-10 00:00:00 , DOI: 10.1021/acs.jchemed.8b00407 David R. McMillin 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-08-10 00:00:00 , DOI: 10.1021/acs.jchemed.8b00407 David R. McMillin 1
Affiliation
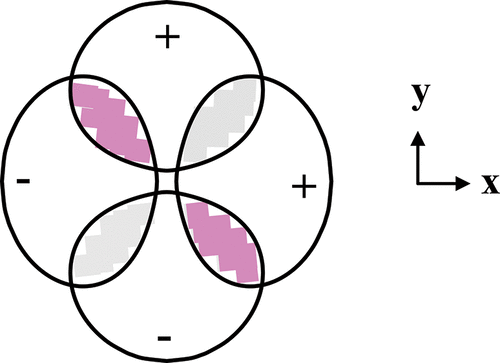
|
The Pauli exclusion principle is a key postulate of the quantum theory and informs much of what we know about matter. In terms of electronic structure, the lone, deceptively simple mathematical requirement is that the total wave function be antisymmetric with respect to the exchange of any two electrons. However, visualizing the effect on the electron distribution presents a formidable challenge to students because of the complexity and number of variables involved. Approaches to gaining insight developed here include a quantitative analysis of two-dimensional configuration spaces as well as a qualitative assessment of interference patterns. For 2px12py1 states as representative examples, the analysis reveals that the Pauli principle directs electrons with opposing spins to codistribute along axes passing through the nucleus in the plane of the occupied orbitals. On the other hand, electrons with the same spin are never simultaneously in the same place or on the same axis; instead, they preferentially distribute in perpendicular directions.
中文翻译:

保利原理:对通过轨道交叠透镜看到的波函数的影响
保利排斥原理是量子理论的关键假设,它为我们提供了许多有关物质的知识。在电子结构方面,唯一的,看似简单的数学要求是总波函数相对于任意两个电子的交换是反对称的。然而,由于涉及到的变量的复杂性和数量,对电子分布的可视化对学生提出了巨大的挑战。在这里获得见识的方法包括对二维配置空间的定量分析以及对干扰模式的定性评估。对于2p x 1 2p y 1以状态为代表的例子,分析表明,保利原理引导电子以相反的自旋,沿在占据轨道平面内穿过核的轴共分布。另一方面,具有相同自旋的电子永远不会同时位于同一位置或同一轴上。相反,它们优先沿垂直方向分布。
更新日期:2018-08-10
中文翻译:

保利原理:对通过轨道交叠透镜看到的波函数的影响
保利排斥原理是量子理论的关键假设,它为我们提供了许多有关物质的知识。在电子结构方面,唯一的,看似简单的数学要求是总波函数相对于任意两个电子的交换是反对称的。然而,由于涉及到的变量的复杂性和数量,对电子分布的可视化对学生提出了巨大的挑战。在这里获得见识的方法包括对二维配置空间的定量分析以及对干扰模式的定性评估。对于2p x 1 2p y 1以状态为代表的例子,分析表明,保利原理引导电子以相反的自旋,沿在占据轨道平面内穿过核的轴共分布。另一方面,具有相同自旋的电子永远不会同时位于同一位置或同一轴上。相反,它们优先沿垂直方向分布。











































 京公网安备 11010802027423号
京公网安备 11010802027423号