当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
SpyTag/SpyCatcher Functionalization of E2 Nanocages with Stimuli-Responsive Z-ELP Affinity Domains for Tunable Monoclonal Antibody Binding and Precipitation Properties
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-08-10 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00458 Andrew R. Swartz 1 , Wilfred Chen 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-08-10 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00458 Andrew R. Swartz 1 , Wilfred Chen 1
Affiliation
|
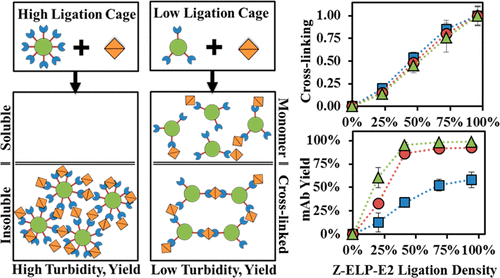
E2 nanocages functionalized with Z-domain-elastin-like polypeptide affinity ligands (Z-ELP40) using Sortase A (SrtA) ligation have been shown to be a promising scaffold for purifying monoclonal antibodies (mAbs) based on affinity precipitation. However, the reversible nature of SrtA reaction has been attributed to the low ligation efficiency (<25%) and has significantly limited the practical utility of the technology. Here, we reported an improved conjugation platform using the SpyTag/SpyCatcher pair to form a spontaneous isopeptide bond between SpyTag-E2 and Z-ELP-SpyCatcher fusion proteins of two different ELP chain-lengths. Using this system, E2 ligation efficiencies exceeding 90% were obtained with both 40- and 80-repeat Z-ELP-SpyCatcher fusions. This enabled the production of nanocages fully functionalized with Z-ELP for improved aggregation and mAb binding. Compared to the 50% decorated Z-ELP40-E2 nanocages produced by SrtA ligation, the fully decorated Z-ELP80-Spy-E2 nanocages exhibited a 10 °C lower transition temperature and a 2-fold higher mAb binding capacity. The improved transition property of the longer Z-ELP80 backbone allowed for >90% recovery of Z-ELP80-Spy-E2 nanocages at room temperature using 0.1 M ammonium sulfate after mAb elution. The flexibility of customizing different affinity domains onto the SpyTag-E2 scaffold should expand our ability to purify other non-mAb target proteins based on affinity precipitation.
中文翻译:

E2纳米笼的SpyTag / SpyCatcher功能化,具有可响应的Z-ELP亲和力域,可调节的单克隆抗体结合和沉淀特性
用Z结构域弹性蛋白样多肽亲和配体功能化的E2纳米笼(Z-ELP 40)使用分选酶A(SrtA)的连接已被证明是一种基于亲和沉淀来纯化单克隆抗体(mAb)的有前途的支架。但是,SrtA反应的可逆性归因于低的连接效率(<25%),并极大地限制了该技术的实用性。在这里,我们报告了一个改进的共轭平台,使用SpyTag / SpyCatcher对在两个不同ELP链长的SpyTag-E2和Z-ELP-SpyCatcher融合蛋白之间形成自发的异肽键。使用该系统,通过40和80重复的Z-ELP-SpyCatcher融合体,均可获得超过90%的E2连接效率。这使得能够生产用Z-ELP完全功能化的纳米笼,以改善聚集和mAb结合。相比于装饰有50%的Z-ELP 40通过SrtA连接产生的-E2纳米笼子,完全装饰的Z-ELP 80 -Spy-E2纳米笼子的转变温度低10°C,mAb结合能力高2倍。较长的Z-ELP 80骨架的改进的过渡性能允许在mAb洗脱后使用0.1 M硫酸铵在室温下> 90%回收Z-ELP 80 -Spy-E2纳米笼。在SpyTag-E2支架上自定义不同亲和结构域的灵活性应扩展我们基于亲和沉淀纯化其他非mAb目标蛋白的能力。
更新日期:2018-08-10
中文翻译:

E2纳米笼的SpyTag / SpyCatcher功能化,具有可响应的Z-ELP亲和力域,可调节的单克隆抗体结合和沉淀特性
用Z结构域弹性蛋白样多肽亲和配体功能化的E2纳米笼(Z-ELP 40)使用分选酶A(SrtA)的连接已被证明是一种基于亲和沉淀来纯化单克隆抗体(mAb)的有前途的支架。但是,SrtA反应的可逆性归因于低的连接效率(<25%),并极大地限制了该技术的实用性。在这里,我们报告了一个改进的共轭平台,使用SpyTag / SpyCatcher对在两个不同ELP链长的SpyTag-E2和Z-ELP-SpyCatcher融合蛋白之间形成自发的异肽键。使用该系统,通过40和80重复的Z-ELP-SpyCatcher融合体,均可获得超过90%的E2连接效率。这使得能够生产用Z-ELP完全功能化的纳米笼,以改善聚集和mAb结合。相比于装饰有50%的Z-ELP 40通过SrtA连接产生的-E2纳米笼子,完全装饰的Z-ELP 80 -Spy-E2纳米笼子的转变温度低10°C,mAb结合能力高2倍。较长的Z-ELP 80骨架的改进的过渡性能允许在mAb洗脱后使用0.1 M硫酸铵在室温下> 90%回收Z-ELP 80 -Spy-E2纳米笼。在SpyTag-E2支架上自定义不同亲和结构域的灵活性应扩展我们基于亲和沉淀纯化其他非mAb目标蛋白的能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号