Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-08-10 , DOI: 10.1016/j.jfluchem.2018.08.004 Federico Salsi , Gisele Bulhões Portapilla , Konstantin Schutjajew , Zumira Aparecida Carneiro , Adelheid Hagenbach , Sérgio de Albuquerque , Pedro Ivo da Silva Maia , Ulrich Abram
|
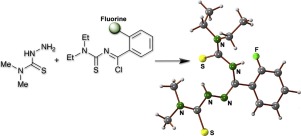
A series of thiosemicarbazones was obtained by condensation of halogenated N-(diethylaminothiocarbonyl)benzimidoyl chlorides (3b–3h) with 4,4-dimethyl-3-thiosemicarbazide. The activity of the halogenated compounds against the parasite Trypanosoma cruzi was evaluated and compared to the previously reported activity of the corresponding non-substituted thiosemicarbazone. It was found that the halogen-substitution enhances in most cases the anti-parasitic activity. The meta-fluorinated compound (4g) was identified as the most potent one (IC50= 9.0 μM, CC50 > 200 μM), having a selectivity index (SI = IC50/CC50), which is 4-times higher than that of the non-substituted compound. Slight modification of the reaction conditions employed for the synthesis of some of the benzoylthioureas 3a–3g led to the unexpected formation of novel halogenated 6-amino-1,3,5-thiadiazine-2-thiones.
中文翻译:

氟化苯甲酰硫脲衍生的硫代氨基脲和噻二嗪:合成,晶体结构和抗克氏锥虫活性
通过将卤化的N-(二乙基氨基硫代羰基)苯甲酰氯(3b–3h)与4,4-二甲基-3-硫代氨基脲缩合,可获得一系列的硫代氨基脲。评估了卤代化合物对寄生虫克氏锥虫的活性,并将其与先前报道的相应的未取代的硫代半脲的活性进行了比较。发现在大多数情况下卤素取代增强了抗寄生虫活性。所述元-fluorinated化合物(4克)被确定为最有效的一个(IC 50 = 9.0μM,CC 50 > 200μM),具有选择性指数(SI = IC 50 / CC 50),比未取代的化合物高4倍。合成某些苯甲酰硫脲3a-3g所用反应条件的轻微改变导致意外形成了新型卤代6-氨基-1,3,5-噻二嗪-2-硫酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号