Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2018-08-10 , DOI: 10.1016/j.jhazmat.2018.08.029 Zhengyang Gao , Minghui Li , Yao Sun , Weijie Yang
|
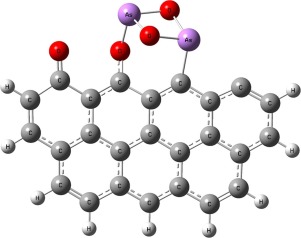
The adsorption mechanism of As2O3 on carbonaceous surface modified with oxygen functional complexes was studied using density functional theory to understand the effect of oxygen functional complexes on arsenic adsorption. Full-parameter geometrical optimization and single point energy were calculated on B3LYP/def2-SVP and B3LYP/def2-TZVP level. Results showed that As2O3 adsorption on bare carbonaceous surface took place in physical as well as chemical way. The adsorption energies were between -2.07 kJ/mol to -480.20 kJ/mol. Compared to armchair model, zigzag model was more suitable as a carbonaceous sorbent. The participation of oxygen functional complexes greatly promoted the surface activity of carbonaceous surface and its adsorption capacity on arsenic. The adsorption energies of arsenic on carbonaceous surface with oxygen functional complexes were between -111.56 kJ/mol to -669.46 kJ/mol. The promotion order of oxygen functional complexes on surface activity was: phenol > lactone > carbonyl > semiquinone > carboxyl. Oxygen functional complexes promoted adsorption capacity of carbonaceous surface through enhancing the activities of neighboring carbon atoms rather than directly providing active sites. Mayer bond order was a reliable way to understand the adsorption process of arsenic on carbonaceous surface. This study provides a new idea for using modified carbonaceous sorbent to remove arsenic pollution from power stations.
中文翻译:

含氧官能团对碳表面砷吸附的影响
利用密度泛函理论研究了As 2 O 3在含氧官能团修饰的碳质表面上的吸附机理,以了解氧官能团对砷吸附的影响。在B3LYP / def2-SVP和B3LYP / def2-TZVP级别上计算了全参数几何优化和单点能量。结果表明,As 2 O 3在裸露的碳质表面上的吸附以物理和化学方式发生。吸附能在-2.07 kJ / mol至-480.20 kJ / mol之间。与扶手椅模型相比,之字形模型更适合用作碳质吸附剂。氧官能团的参与极大地促进了碳质表面的表面活性及其对砷的吸附能力。含氧官能团的砷在碳质表面上的吸附能在-111.56 kJ / mol至-669.46 kJ / mol之间。氧官能团配合物对表面活性的促进顺序为:苯酚>内酯>羰基>半醌>羧基。氧官能团配合物通过增强相邻碳原子的活性而不是直接提供活性位而提高了碳质表面的吸附能力。Mayer键序是了解砷在碳质表面上吸附过程的可靠方法。这项研究为使用改性碳质吸附剂去除发电厂的砷污染提供了新思路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号