当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Analysis of Syngas Production and Sulfur Capturing from a Mixture of Methane and Hydrogen Sulfide Using a Solar Thermochemical Redox Cycle
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-08-21 , DOI: 10.1021/acs.iecr.8b02484 Abhishek Singh 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-08-21 , DOI: 10.1021/acs.iecr.8b02484 Abhishek Singh 1
Affiliation
|
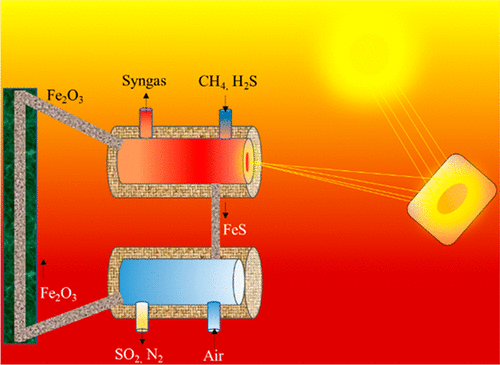
Syngas production and sulfur capturing from a mixture of methane and H2S via a novel two-step solar thermochemical cycle based on metal oxide/metal sulfide redox reactions is thermodynamically analyzed. Fe2O3/FeS is used as a model metal oxide/metal sulfide pair for this study. During the reduction step, Fe2O3 is reduced to FeS using a mixture of CH4 and H2S. In this process, syngas (CO + H2) is produced in the gas phase. In the oxidation step, FeS is oxidized using air to obtain Fe2O3 in the solid phase and SO2 and unreacted N2 in the gas phase. The produced SO2 can be used to generate sulfuric acid. Favorable operating conditions for the redox cycle are determined using an open system, thermodynamic model. For the reduction step, T = 1200 K at 1 bar pressure provides complete conversion of Fe2O3 to FeS. At these conditions, gas phase products contain mainly H2 and CO. Carbon formation is also predicted by the model between 580 and 1150 K temperatures. Thermodynamic analysis shows complete conversion of FeS to Fe2O3 during the oxidation step at T ≥ 600 K and 1 bar pressure. Effect of higher system pressures over the Fe2O3 reduction process is also determined using the model. With the increase in system pressure, carbon formation decreases. At 10 bar system pressure, no carbon formation is predicted by the thermodynamic model. A thermodynamic process model is also developed to assess the energetic feasibility of the complete process. Concentrated solar power can be used to provide the necessary energy for the endothermic Fe2O3 reduction process. Effect of concentration ratio and heat recuperation from exhaust gases over solar to fuel energy efficiency is studied. Energy efficiencies of the complete process at various oxidation temperatures are also determined using the thermodynamic process model. An overall system efficiency of 46.9% can be achieved for heat exchanger effectiveness of 0.9 and concentration ratio of 1000 when reduction and oxidation reactions are performed at 1200 K temperature.
中文翻译:

利用太阳热化学氧化还原循环对甲烷和硫化氢混合物生产合成气和捕获硫的热力学分析
热力学分析了基于金属氧化物/金属硫化物氧化还原反应的新颖的两步太阳能热化学循环,从甲烷和H 2 S的混合物中合成气的生产和硫的捕集。Fe 2 O 3 / FeS用作本研究的模型金属氧化物/金属硫化物对。在还原步骤中,使用CH 4和H 2 S的混合物将Fe 2 O 3还原为FeS 。在此过程中,在气相中生成合成气(CO + H 2)。在氧化步骤中,使用空气氧化FeS,以获得固相中的Fe 2 O 3和SO 2和未反应的N 2。在气相中。产生的SO 2可用于产生硫酸。使用开放系统热力学模型确定氧化还原循环的适宜操作条件。对于还原步骤,在1 bar压力下T = 1200 K可将Fe 2 O 3完全转化为FeS。在这些条件下,气相产物主要包含H 2和CO。该模型还预测了580至1150 K温度之间的碳形成。热力学分析节目的FeS的完全转化成Fe 2 ö 3期间,在氧化步骤Ť ≥600 K和1吨的压力。较高的系统压力对Fe 2 O的影响使用模型还可以确定3个还原过程。随着系统压力的增加,碳的形成减少。在10 bar系统压力下,热力学模型无法预测碳的形成。还开发了一个热力学过程模型来评估整个过程的能量可行性。可以使用集中的太阳能为吸热的Fe 2 O 3提供必要的能量还原过程。研究了废气中的浓度比和热回收对太阳能效率的影响。还可以使用热力学过程模型确定在各种氧化温度下整个过程的能量效率。当在1200 K温度下进行还原和氧化反应时,换热器效率为0.9,浓缩比为1000时,系统总效率为46.9%。
更新日期:2018-08-21
中文翻译:

利用太阳热化学氧化还原循环对甲烷和硫化氢混合物生产合成气和捕获硫的热力学分析
热力学分析了基于金属氧化物/金属硫化物氧化还原反应的新颖的两步太阳能热化学循环,从甲烷和H 2 S的混合物中合成气的生产和硫的捕集。Fe 2 O 3 / FeS用作本研究的模型金属氧化物/金属硫化物对。在还原步骤中,使用CH 4和H 2 S的混合物将Fe 2 O 3还原为FeS 。在此过程中,在气相中生成合成气(CO + H 2)。在氧化步骤中,使用空气氧化FeS,以获得固相中的Fe 2 O 3和SO 2和未反应的N 2。在气相中。产生的SO 2可用于产生硫酸。使用开放系统热力学模型确定氧化还原循环的适宜操作条件。对于还原步骤,在1 bar压力下T = 1200 K可将Fe 2 O 3完全转化为FeS。在这些条件下,气相产物主要包含H 2和CO。该模型还预测了580至1150 K温度之间的碳形成。热力学分析节目的FeS的完全转化成Fe 2 ö 3期间,在氧化步骤Ť ≥600 K和1吨的压力。较高的系统压力对Fe 2 O的影响使用模型还可以确定3个还原过程。随着系统压力的增加,碳的形成减少。在10 bar系统压力下,热力学模型无法预测碳的形成。还开发了一个热力学过程模型来评估整个过程的能量可行性。可以使用集中的太阳能为吸热的Fe 2 O 3提供必要的能量还原过程。研究了废气中的浓度比和热回收对太阳能效率的影响。还可以使用热力学过程模型确定在各种氧化温度下整个过程的能量效率。当在1200 K温度下进行还原和氧化反应时,换热器效率为0.9,浓缩比为1000时,系统总效率为46.9%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号