Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-08-10 , DOI: 10.1016/j.jinorgbio.2018.08.008 Martina Quaretti , Marina Porchia , Francesco Tisato , Angela Trapananti , Giuliana Aquilanti , Marko Damjanović , Luciano Marchiò , Marco Giorgetti , Matteo Tegoni
|
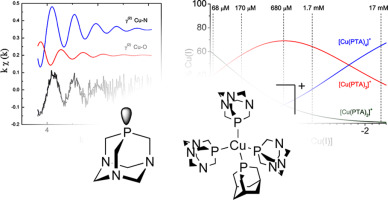
The chemistry of copper(I) with water-soluble phosphines is an emergent area of study which has the objective of finding ligands that stabilize copper in its lower oxidation state. Cu(I) has been found relevant in the mechanism of copper transports into cells, and the accessibility of this oxidation state has implications in oxidative stress processes. For these reasons the possibility to deal with stable, water soluble copper(I) is an attractive approach for devising new biologically relevant metal-based drugs and chelating agents. Here we present the X-ray absorption spectroscopy (XAS) and UV–visible spectrophotometric study of the [Cu(PTA)4]BF4 complex (PTA = aminophosphine‑1,3,5‑triaza‑7‑phosphaadamantane). In particular, we have studied the stability of the [Cu(PTA)n]+ species (n = 2–4) in aqueous medium, and their speciation as a function of the total [Cu(PTA)4]BF4 concentration by means of competitive UV–visible spectrophotometric titrations using metallochromic indicators. Also, the structure in solution of the Cu(I)/PTA species and the nature of the first coordination sphere of the metal were studied by transformed XAS. Both techniques allowed to study samples with total [Cu(PTA)4]BF4 concentration down to 68–74 μM, possibly relevant for biological applications. Overall, our data suggest that the [Cu(PTA)n]+ species are stable in solution, among which [Cu(PTA)2]+ has a remarkable thermodynamic stability. The tendency of this last complex to form adducts with N-donor ligands is demonstrated by the spectrophotometric data. The biological relevance of PTA towards Cu(I), especially in terms of chemotreatments and chelation therapy, is discussed on the basis of the speciation model the Cu(I)/PTA system.
中文翻译:

[Cu(PTA)4 ] +配合物(PTA =氨基膦-1,3,5-三氮杂-7-磷金刚烷)在水溶液中的热力学稳定性和结构
铜(I)与水溶性膦的化学反应是一个新兴的研究领域,其目的是寻找能将铜稳定在较低氧化态的配体。已经发现Cu(I)与铜向细胞中的迁移机制有关,并且该氧化态的可及性对氧化应激过程具有影响。由于这些原因,处理稳定的水溶性铜(I)的可能性是设计新的生物学相关的基于金属的药物和螯合剂的有吸引力的方法。在这里,我们介绍了[Cu(PTA)4 ] BF 4络合物(PTA =氨基膦-1,3,5-三氮杂-7-磷基金刚烷)的X射线吸收光谱(XAS)和紫外可见分光光度法研究。特别是,我们研究了[Cu(PTA)n] +种类(n = 2-4)在水介质中,以及它们的形态通过使用金属致变色指示剂的竞争性UV-可见分光光度滴定法确定为[Cu(PTA)4 ] BF 4总浓度的函数。此外,通过转化XAS研究了Cu(I)/ PTA物种在溶液中的结构以及金属的第一配位球的性质。两种技术都允许研究总[Cu(PTA)4 ] BF 4浓度低至68–74μM的样品,这可能与生物学应用有关。总体而言,我们的数据表明[Cu(PTA)n ] +物种在溶液中是稳定的,其中[Cu(PTA)2 ] +具有卓越的热力学稳定性。分光光度数据证明了该最后的配合物与N-供体配体形成加合物的趋势。在形态模型Cu(I)/ PTA系统的基础上,讨论了PTA对Cu(I)的生物学相关性,特别是在化学治疗和螯合治疗方面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号