当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Energy‐Efficient Nitrogen Reduction to Ammonia at Low Overpotential in Aqueous Electrolyte under Ambient Conditions
ChemSusChem ( IF 8.4 ) Pub Date : 2018-09-04 , DOI: 10.1002/cssc.201801632 Dabin Wang 1 , Luis Miguel Azofra 2 , Moussab Harb 2 , Luigi Cavallo 2 , Xinyi Zhang 1 , Bryan H. R. Suryanto 1 , Douglas R. MacFarlane 1
ChemSusChem ( IF 8.4 ) Pub Date : 2018-09-04 , DOI: 10.1002/cssc.201801632 Dabin Wang 1 , Luis Miguel Azofra 2 , Moussab Harb 2 , Luigi Cavallo 2 , Xinyi Zhang 1 , Bryan H. R. Suryanto 1 , Douglas R. MacFarlane 1
Affiliation
|
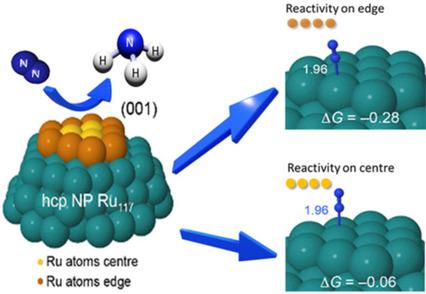
The electrochemical nitrogen reduction reaction (NRR) under ambient conditions is a promising alternative to the traditional energy‐intensive Haber–Bosch process to produce NH3. The challenge is to achieve a sufficient energy efficiency, yield rate, and selectivity to make the process practical. Here, we demonstrate that Ru nanoparticles (NPs) enable NRR in 0.01 m HCl aqueous solution at very high energy efficiency, that is, very low overpotentials. Remarkably, the NRR occurs at a potential close to or even above the H+/H2 reversible potential, significantly enhancing the NRR selectivity versus the production of H2. NH3 yield rates as high as ≈5.5 mg h−1 m−2 at 20 °C and 21.4 mg h−1 m−2 at 60 °C were achieved at a redox potential (E) of −100 mV versus the reversible hydrogen electrode (RHE), whereas a highest Faradaic efficiency (FE) of ≈5.4 % is achievable at E=+10 mV vs. RHE. This work demonstrates the potential use of Ru NPs as an efficient catalyst for NRR at ambient conditions. This ability to catalyze NRR at potentials near or above RHE is imperative in improving the NRR selectivity towards a practical process as well as rendering the H2 viable as byproduct. Density functional theory calculations of the mechanism suggest that the efficient NRR process occurring on these predominantly Ru (0 0 1) surfaces is catalyzed by a dissociative mechanism.
中文翻译:

在环境条件下在低电位下将电解质高效节能地还原为氨
在环境条件下进行电化学氮还原反应(NRR)是一种可替代传统能源密集型Haber-Bosch工艺生产NH 3的有前途的方法。挑战在于如何获得足够的能源效率,产率和选择性,以使该工艺切实可行。在这里,我们证明了Ru纳米颗粒(NPs)能够以非常高的能量效率(即非常低的超电势)在0.01 m HCl水溶液中实现NRR 。值得注意的是,NRR在接近或什至高于H + / H 2可逆电位的电位下发生,与H 2的产生相比,显着提高了NRR的选择性。NH 3产率高达≈5.5mg h -1 m-2在20℃和21.4毫克ħ -1 米-2在60℃物在氧化还原电势(实现Ë的)- 100毫伏相对于可逆氢电极(RHE),而最高法拉第效率(FE)的与RHE相比,在E = + 10 mV时可达到约5.4%。这项工作证明了Ru NPs在环境条件下作为NRR的有效催化剂的潜在用途。在接近或高于RHE的电位上催化NRR的能力对于提高NRR对实际工艺的选择性以及使H 2转化为必不可少的可行的副产品。该机理的密度泛函理论计算表明,在这些主要是Ru(0 0 1)表面上发生的有效NRR过程是由分解机理催化的。
更新日期:2018-09-04
中文翻译:

在环境条件下在低电位下将电解质高效节能地还原为氨
在环境条件下进行电化学氮还原反应(NRR)是一种可替代传统能源密集型Haber-Bosch工艺生产NH 3的有前途的方法。挑战在于如何获得足够的能源效率,产率和选择性,以使该工艺切实可行。在这里,我们证明了Ru纳米颗粒(NPs)能够以非常高的能量效率(即非常低的超电势)在0.01 m HCl水溶液中实现NRR 。值得注意的是,NRR在接近或什至高于H + / H 2可逆电位的电位下发生,与H 2的产生相比,显着提高了NRR的选择性。NH 3产率高达≈5.5mg h -1 m-2在20℃和21.4毫克ħ -1 米-2在60℃物在氧化还原电势(实现Ë的)- 100毫伏相对于可逆氢电极(RHE),而最高法拉第效率(FE)的与RHE相比,在E = + 10 mV时可达到约5.4%。这项工作证明了Ru NPs在环境条件下作为NRR的有效催化剂的潜在用途。在接近或高于RHE的电位上催化NRR的能力对于提高NRR对实际工艺的选择性以及使H 2转化为必不可少的可行的副产品。该机理的密度泛函理论计算表明,在这些主要是Ru(0 0 1)表面上发生的有效NRR过程是由分解机理催化的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号