当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Recognition of a Thomsen–Friedenreich Antigen Mimetic Targeting Human Galectin‐3
ChemMedChem ( IF 3.6 ) Pub Date : 2018-09-11 , DOI: 10.1002/cmdc.201800525 Sabrina Santarsia 1 , Ana Sofia Grosso 2 , Filipa Trovão 2 , Jesús Jiménez-Barbero 3, 4, 5 , Ana Luísa Carvalho 2 , Cristina Nativi 1 , Filipa Marcelo 2
ChemMedChem ( IF 3.6 ) Pub Date : 2018-09-11 , DOI: 10.1002/cmdc.201800525 Sabrina Santarsia 1 , Ana Sofia Grosso 2 , Filipa Trovão 2 , Jesús Jiménez-Barbero 3, 4, 5 , Ana Luísa Carvalho 2 , Cristina Nativi 1 , Filipa Marcelo 2
Affiliation
|
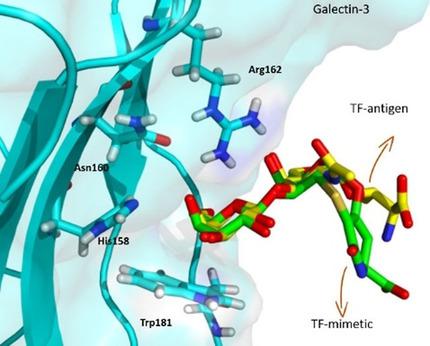
Overexpression of the Thomsen–Friedenreich (TF) antigen in cell membrane proteins occurs in 90 % of adenocarcinomas. Additionally, the binding of the TF antigen to human galectin‐3 (Gal‐3), also frequently overexpressed in malignancy, promotes cancer progression and metastasis. In this context, structures that interfere with this specific interaction have the potential to prevent cancer metastasis. A multidisciplinary approach combining the optimized synthesis of a TF antigen mimetic with NMR, X‐ray crystallography methods, and isothermal titration calorimetry assays was used to unravel the molecular structural details that govern the Gal‐3/TF mimetic interaction. The TF mimetic has a binding affinity for Gal‐3 similar to that of the TF natural antigen and retains the binding epitope and bioactive conformation observed for the native antigen. Furthermore, from a thermodynamic perspective, a decrease in the enthalpic contribution was observed for the Gal‐3/TF mimetic complex; however, this behavior is compensated by a favorable gain in entropy. From a structural perspective, these results establish our TF mimetic as a scaffold to design multivalent solutions to potentially interfere with Gal‐3 aberrant interactions and for likely use in hampering Gal‐3‐mediated cancer cell adhesion and metastasis.
中文翻译:

针对人Galectin-3的Thomsen-Friedenreich抗原模拟物的分子识别。
90%的腺癌中细胞膜蛋白中的Thomsen-Friedenreich(TF)抗原过度表达。此外,TF抗原与人半乳糖凝集素3(Gal-3)的结合(通常在恶性肿瘤中也经常过表达)会促进癌症的发展和转移。在这种情况下,干扰这种特定相互作用的结构具有预防癌症转移的潜力。采用多学科方法将TF抗原模拟物的优化合成与NMR,X射线晶体学方法和等温滴定热分析法相结合,以揭示控制Gal-3 / TF模拟物相互作用的分子结构细节。TF模拟物对Gal-3具有与TF天然抗原相似的结合亲和力,并保留了针对天然抗原观察到的结合表位和生物活性构象。此外,从热力学角度看,Gal-3 / TF模拟复合物的焓贡献降低。然而,这种行为被熵的有利增益所补偿。从结构的角度来看,这些结果使我们的TF模拟物成为了一种支架,可以设计多价解决方案来潜在干扰Gal-3异常相互作用,并有可能用于阻止Gal-3介导的癌细胞粘附和转移。
更新日期:2018-09-11
中文翻译:

针对人Galectin-3的Thomsen-Friedenreich抗原模拟物的分子识别。
90%的腺癌中细胞膜蛋白中的Thomsen-Friedenreich(TF)抗原过度表达。此外,TF抗原与人半乳糖凝集素3(Gal-3)的结合(通常在恶性肿瘤中也经常过表达)会促进癌症的发展和转移。在这种情况下,干扰这种特定相互作用的结构具有预防癌症转移的潜力。采用多学科方法将TF抗原模拟物的优化合成与NMR,X射线晶体学方法和等温滴定热分析法相结合,以揭示控制Gal-3 / TF模拟物相互作用的分子结构细节。TF模拟物对Gal-3具有与TF天然抗原相似的结合亲和力,并保留了针对天然抗原观察到的结合表位和生物活性构象。此外,从热力学角度看,Gal-3 / TF模拟复合物的焓贡献降低。然而,这种行为被熵的有利增益所补偿。从结构的角度来看,这些结果使我们的TF模拟物成为了一种支架,可以设计多价解决方案来潜在干扰Gal-3异常相互作用,并有可能用于阻止Gal-3介导的癌细胞粘附和转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号