当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cargo Retention inside P22 Virus-Like Particles
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acs.biomac.8b00867 Kimberly McCoy 1 , Ekaterina Selivanovitch 1 , Daniel Luque 2, 3 , Byeongdu Lee 4 , Ethan Edwards 1 , José R. Castón 2 , Trevor Douglas 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acs.biomac.8b00867 Kimberly McCoy 1 , Ekaterina Selivanovitch 1 , Daniel Luque 2, 3 , Byeongdu Lee 4 , Ethan Edwards 1 , José R. Castón 2 , Trevor Douglas 1
Affiliation
|
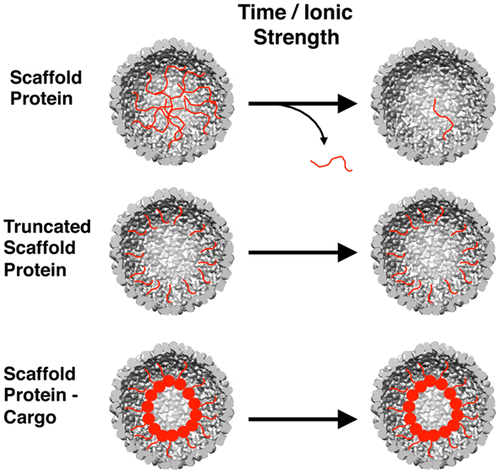
Viral protein cages, with their regular and programmable architectures, are excellent platforms for the development of functional nanomaterials. The ability to transform a virus into a material with intended structure and function relies on the existence of a well-understood model system, a noninfectious virus-like particle (VLP) counterpart. Here, we study the factors important to the ability of P22 VLP to retain or release various protein cargo molecules depending on the nature of the cargo, the capsid morphology, and the environmental conditions. Because the interaction between the internalized scaffold protein (SP) and the capsid coat protein (CP) is noncovalent, we have studied the efficiency with which a range of SP variants can dissociate from the interior of different P22 VLP morphologies and exit by traversing the porous capsid. Understanding the types of cargos that are either retained or released from the P22 VLP will aid in the rational design of functional nanomaterials.
中文翻译:

P22病毒样颗粒内部的货物滞留
病毒蛋白笼子具有常规和可编程的结构,是开发功能纳米材料的绝佳平台。将病毒转化为具有预期结构和功能的材料的能力取决于是否存在一个众所周知的模型系统,即非传染性病毒样颗粒(VLP)对应物。在这里,我们根据货物的性质,衣壳形态和环境条件,研究了影响P22 VLP保留或释放各种蛋白质货物分子的能力的重要因素。因为内在化的支架蛋白(SP)与衣壳蛋白(CP)之间的相互作用是非共价的,所以我们研究了一系列SP变体可以从不同的P22 VLP形态内部解离并通过遍历多孔结构退出的效率衣壳。
更新日期:2018-08-09
中文翻译:

P22病毒样颗粒内部的货物滞留
病毒蛋白笼子具有常规和可编程的结构,是开发功能纳米材料的绝佳平台。将病毒转化为具有预期结构和功能的材料的能力取决于是否存在一个众所周知的模型系统,即非传染性病毒样颗粒(VLP)对应物。在这里,我们根据货物的性质,衣壳形态和环境条件,研究了影响P22 VLP保留或释放各种蛋白质货物分子的能力的重要因素。因为内在化的支架蛋白(SP)与衣壳蛋白(CP)之间的相互作用是非共价的,所以我们研究了一系列SP变体可以从不同的P22 VLP形态内部解离并通过遍历多孔结构退出的效率衣壳。











































 京公网安备 11010802027423号
京公网安备 11010802027423号