当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mycobacterial MenJ: An Oxidoreductase Involved in Menaquinone Biosynthesis
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acschembio.8b00402 Ashutosh Upadhyay 1 , Santosh Kumar 1 , Steven A. Rooker 1 , Jordan T. Koehn 2 , Debbie C. Crans 2 , Michael R. McNeil 1 , J. Shaun Lott 3 , Dean C. Crick 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acschembio.8b00402 Ashutosh Upadhyay 1 , Santosh Kumar 1 , Steven A. Rooker 1 , Jordan T. Koehn 2 , Debbie C. Crans 2 , Michael R. McNeil 1 , J. Shaun Lott 3 , Dean C. Crick 1
Affiliation
|
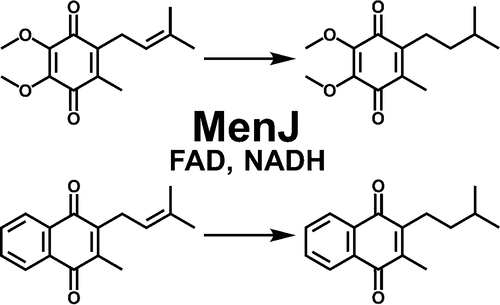
MenJ, annotated as an oxidoreductase, was recently demonstrated to catalyze the reduction (saturation) of a single double bond in the isoprenyl side-chain of mycobacterial menaquinone. This modification was shown to be essential for bacterial survival in J774A.1 macrophage-like cells, suggesting that MenJ may be a conditional drug target in Mycobacterium tuberculosis and other pathogenic mycobacteria. Recombinant protein was expressed in a heterologous host, and the activity was characterized. Although highly regiospecific in vivo, the activity is not absolutely regiospecific in vitro; in addition, the enzyme is not specific for naphthoquinones vs benzoquinones. Coenzyme Q-1 (a benzoquinone, UQ-1) was used as the lipoquinone substrate, and NADH oxidation was followed spectrophotometrically as the activity readout. NADPH could not be substituted for NADH in the reaction mixture. The enzyme contains a FAD binding site that was 72% occupied in the purified recombinant protein. Enzyme activity was maximal at 37 °C and pH 7.0; addition of divalent cations, EDTA, and reducing agents such as dithiothreitol to the reaction mixture had no effect on activity. The addition of detergents did not stimulate activity, and addition of saturating levels of FAD had relatively little effect on the observed kinetic parameters. These properties allowed the development of a facile assay needed to study this potential drug target, which is also amenable to high throughput screening. The Km values for UQ-1 using recombinant MenJ from Mycobacterium smegmatis or M. tuberculosis without saturating concentrations of FAD were found to be 52 ± 9.6 and 44 ± 4.8 μM, respectively, while the KmNADH values were determined to be 59 ± 14 and 64 ± 15 μM. The Km for MK-1, the menaquinone analogue of UQ-1, using recombinant MenJ from M. tuberculosis without saturating concentrations of FAD but in the presence of 0.5% Tween 80 was shown to be 30 ± 2.9 μM. Thus, this is the first report of a kinetic characterization of a member of the geranylgeranyl reductase family of enzymes.
中文翻译:

分枝杆菌MenJ:氧化还原酶参与甲萘醌的生物合成
MenJ,被标注为氧化还原酶,最近被证明可以催化分枝杆菌甲基萘醌异戊二烯侧链中单个双键的还原(饱和)。已表明这种修饰对于J774A.1巨噬细胞样细胞中细菌存活至关重要,这表明MenJ可能是结核分枝杆菌和其他病原性分枝杆菌中的条件药物靶标。重组蛋白在异源宿主中表达,并对其活性进行了表征。尽管在体内具有很高的区域特异性,但在体外活性并不是绝对的区域特异性。; 另外,该酶对萘醌和苯醌不是特异性的。辅酶Q-1(苯醌,UQ-1)用作脂醌底物,分光光度法跟踪NADH氧化,作为活性读数。NADPH无法代替反应混合物中的NADH。该酶包含一个FAD结合位点,该位点在纯化的重组蛋白中占72%。在37°C和pH 7.0下,酶的活性最大。向反应混合物中添加二价阳离子,EDTA和还原剂如二硫苏糖醇对活性没有影响。添加去污剂不会刺激活性,而添加饱和水平的FAD对观察到的动力学参数影响相对较小。这些特性使得可以开发出一种简便的测定方法来研究这种潜在的药物靶标,这也适用于高通量筛选。这使用耻垢分枝杆菌或结核分枝杆菌的重组MenJ在不饱和FAD浓度下使用重组MenJ测得的UQ-1的K m值分别为52±9.6和44± 4.8μM ,而K m NADH值确定为59±14和64±15μM。使用来自结核分枝杆菌的重组MenJ,未饱和FAD浓度,但在0.5%Tween 80存在下,MK-1(UQ-1的甲基萘醌类似物)的K m显示为30±2.9μM。因此,这是酶的香叶基香叶基还原酶家族成员的动力学表征的首次报道。
更新日期:2018-08-09
中文翻译:

分枝杆菌MenJ:氧化还原酶参与甲萘醌的生物合成
MenJ,被标注为氧化还原酶,最近被证明可以催化分枝杆菌甲基萘醌异戊二烯侧链中单个双键的还原(饱和)。已表明这种修饰对于J774A.1巨噬细胞样细胞中细菌存活至关重要,这表明MenJ可能是结核分枝杆菌和其他病原性分枝杆菌中的条件药物靶标。重组蛋白在异源宿主中表达,并对其活性进行了表征。尽管在体内具有很高的区域特异性,但在体外活性并不是绝对的区域特异性。; 另外,该酶对萘醌和苯醌不是特异性的。辅酶Q-1(苯醌,UQ-1)用作脂醌底物,分光光度法跟踪NADH氧化,作为活性读数。NADPH无法代替反应混合物中的NADH。该酶包含一个FAD结合位点,该位点在纯化的重组蛋白中占72%。在37°C和pH 7.0下,酶的活性最大。向反应混合物中添加二价阳离子,EDTA和还原剂如二硫苏糖醇对活性没有影响。添加去污剂不会刺激活性,而添加饱和水平的FAD对观察到的动力学参数影响相对较小。这些特性使得可以开发出一种简便的测定方法来研究这种潜在的药物靶标,这也适用于高通量筛选。这使用耻垢分枝杆菌或结核分枝杆菌的重组MenJ在不饱和FAD浓度下使用重组MenJ测得的UQ-1的K m值分别为52±9.6和44± 4.8μM ,而K m NADH值确定为59±14和64±15μM。使用来自结核分枝杆菌的重组MenJ,未饱和FAD浓度,但在0.5%Tween 80存在下,MK-1(UQ-1的甲基萘醌类似物)的K m显示为30±2.9μM。因此,这是酶的香叶基香叶基还原酶家族成员的动力学表征的首次报道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号