Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-08-10 , DOI: 10.1016/j.cplett.2018.08.027 Janet E. Del Bene , Ibon Alkorta , M. Merced Montero-Campillo , José Elguero
|
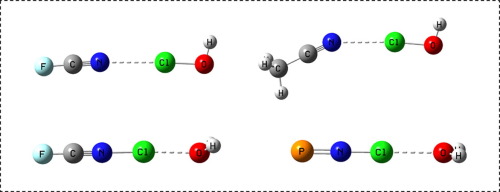
Ab initio MP2/aug’-cc-pVTZ calculations demonstrate that protonation of N-Base:ClOH complexes at O leads to complexes (N-Base-Cl)+:OH2, as a traditional N...Cl halogen bond is replaced by a chlorine-transferred Cl...O bond. For a fixed base, the binding energies of the latter complexes are more than an order of magnitude greater than the former. As a function of the Cl-N distance, EOM-CCSD Cl-N coupling constants show the evolution of the traditional halogen bond in the N-Base:ClOH complexes, to a chlorine-shared halogen bond in N2:ClOH2+, to a covalent bond in (N-Base-Cl)+:OH2. 1J(Cl-N) values for these complexes resemble 1J(Cl-N) for the ions (N-Base-Cl)+.
中文翻译:

使用质子化将N-Base:ClOH络合物中的Cl··N卤素键更改为Cl···O卤素键
从头算MP2 / aug'-cc-pVTZ计算表明,O处N-Base:ClOH配合物的质子化会导致配合物(N-Base-Cl)+:OH 2,因为传统的N ... Cl卤素键已被取代通过氯转移的Cl ... O键 对于固定的碱,后者络合物的结合能比前者大一个数量级。作为Cl-N距离的函数,EOM-CCSD Cl-N耦合常数显示了N-Base:ClOH络合物中传统卤素键向N 2:ClOH 2 +中氯共享的卤素键的演化, (N-碱-Cl)+:OH 2中的共价键。1个这些络合物的J(Cl-N)值类似于离子(N-Base-Cl)+的1 J(Cl-N)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号