当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The chameleonic reactivity of dilithio bis(alkylamido)cyclodiphosph(iii)azanes with chlorophosphines†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1039/c8dt02087f Mathew E. Otang 1, 2, 3, 4 , David Josephson 1, 2, 3, 4 , Todd Duppong 1, 2, 3, 4 , Lothar Stahl 1, 2, 3, 4
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1039/c8dt02087f Mathew E. Otang 1, 2, 3, 4 , David Josephson 1, 2, 3, 4 , Todd Duppong 1, 2, 3, 4 , Lothar Stahl 1, 2, 3, 4
Affiliation
|
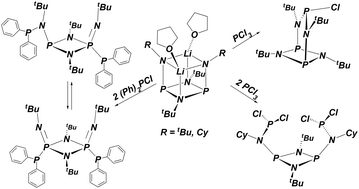
To synthesize a bis(diphosphinylamine) ligand for use in late-metal catalysis dilithio bis(tert-butylamido)cyclodiphosph(III)azane was treated with two equivalents of (Ph)2PCl. The chlorodiphenylphosphine attacked the dilithium salt kinetically at phosphorus, followed by a rearrangement to an unsymmetrical P, N-di-substituted product. Solid-state structures of both products were determined, and the activation energy for the rearrangement was measured. To gain further insight into the location of chlorophosphine attack (N versus P) on lithium diamides, dilithio bis(cyclohexylamido)cyclodiphosph(III)azane or dilithio bis(tert-butylamido)cyclodisilazane was treated with two equivalents of PCl3. In each case only the symmetrically N,N′-substituted bis(PCl2) product was obtained and characterized by X-ray analysis. The structures of these latter compounds are unusual, because in both cases a bicyclic compound with a bis(amido) chelated, central P–Cl moiety was expected based on precedent. The two proximal and juxtaposed PCl2 groups may provide a starting point for potentially novel reactivity studies.
中文翻译:

二硫代双(烷基酰胺基)环二磷(iii)氮烷与氯代膦的chameleonic反应性†
为了合成用于晚金属催化的双(二膦基胺)配体,用两当量的(Ph)2 PCl处理二硫代双(叔丁基氨基)环二磷(III)氮烷。氯二苯膦在磷原子上动力学地攻击了二锂盐,随后重排为不对称的P,N-二取代产物。确定了两种产物的固态结构,并测量了重排的活化能。为了进一步了解氯膦对二酰胺锂,二硫代双(环己酰胺基)环二磷(III)氮杂或二硫代双(叔丁基)的攻击位置(N对P)-丁基酰胺基)环二硅氮烷用两当量的PCl 3处理。在每种情况下,仅获得对称的N,N′-取代的双(PCl 2)产物,并通过X射线分析对其进行表征。后一种化合物的结构是不寻常的,因为根据先例,在这两种情况下均预期具有双(酰胺基)螯合的中心P-Cl部分的双环化合物。这两个近端和并列的PCl 2组可能为潜在的新型反应性研究提供起点。
更新日期:2018-08-07
中文翻译:

二硫代双(烷基酰胺基)环二磷(iii)氮烷与氯代膦的chameleonic反应性†
为了合成用于晚金属催化的双(二膦基胺)配体,用两当量的(Ph)2 PCl处理二硫代双(叔丁基氨基)环二磷(III)氮烷。氯二苯膦在磷原子上动力学地攻击了二锂盐,随后重排为不对称的P,N-二取代产物。确定了两种产物的固态结构,并测量了重排的活化能。为了进一步了解氯膦对二酰胺锂,二硫代双(环己酰胺基)环二磷(III)氮杂或二硫代双(叔丁基)的攻击位置(N对P)-丁基酰胺基)环二硅氮烷用两当量的PCl 3处理。在每种情况下,仅获得对称的N,N′-取代的双(PCl 2)产物,并通过X射线分析对其进行表征。后一种化合物的结构是不寻常的,因为根据先例,在这两种情况下均预期具有双(酰胺基)螯合的中心P-Cl部分的双环化合物。这两个近端和并列的PCl 2组可能为潜在的新型反应性研究提供起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号