当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Evolving State of Continuous Processing in Pharmaceutical API Manufacturing: A Survey of Pharmaceutical Companies and Contract Manufacturing Organizations
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-08-08 00:00:00 , DOI: 10.1021/acs.oprd.8b00160 J. Christopher McWilliams 1 , Ayman D. Allian 2 , Suzanne M. Opalka 3 , Scott A. May 4 , Michel Journet 5 , Timothy M. Braden 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-08-08 00:00:00 , DOI: 10.1021/acs.oprd.8b00160 J. Christopher McWilliams 1 , Ayman D. Allian 2 , Suzanne M. Opalka 3 , Scott A. May 4 , Michel Journet 5 , Timothy M. Braden 4
Affiliation
|
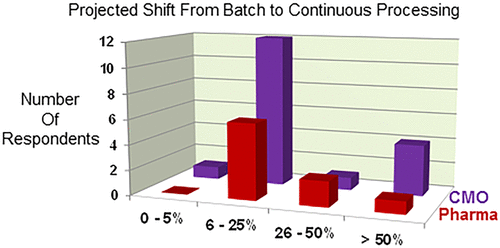
This manuscript provides the results of an in-depth survey assessment of the capabilities, experience, and perspectives on continuous processing in the pharmaceutical sector, with respondents from both pharmaceutical companies and Contract Manufacturing Organizations (CMOs). The survey includes staffing (personnel), chemistry, reaction platforms, postreaction processing, analytical, regulatory, and factors that influence the adoption of continuous manufacturing. The results of the survey demonstrate that the industry has been increasing, and will continue to increase, the portion of total manufacturing executed as continuous processes with a decrease in batch processing. In general, most of the experience with continuous processing on scale have been enabling reaction chemistry, while postprocessing and analytical remain in the very early stages of development and implementation.
中文翻译:

制药原料药制造中连续加工的发展状态:制药公司和合同制造组织的调查
该手稿与制药公司和合同制造组织(CMO)的受访者一起,对制药行业的连续加工能力,经验和观点进行了深入调查评估,得出了结果。该调查包括人员(人员),化学,反应平台,反应后处理,分析,法规和影响采用连续生产的因素。调查结果表明,该行业一直在增加,并将继续增加,以连续过程执行的总制造量在批量处理中有所减少。一般而言,在大规模连续处理方面的大多数经验都可以实现反应化学,
更新日期:2018-08-08
中文翻译:

制药原料药制造中连续加工的发展状态:制药公司和合同制造组织的调查
该手稿与制药公司和合同制造组织(CMO)的受访者一起,对制药行业的连续加工能力,经验和观点进行了深入调查评估,得出了结果。该调查包括人员(人员),化学,反应平台,反应后处理,分析,法规和影响采用连续生产的因素。调查结果表明,该行业一直在增加,并将继续增加,以连续过程执行的总制造量在批量处理中有所减少。一般而言,在大规模连续处理方面的大多数经验都可以实现反应化学,











































 京公网安备 11010802027423号
京公网安备 11010802027423号