Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-08-08 , DOI: 10.1016/j.jinorgbio.2018.08.007 Guowei Xing , Christopher J. Miller , A. Ninh Pham , Adele M. Jones , T. David Waite
|
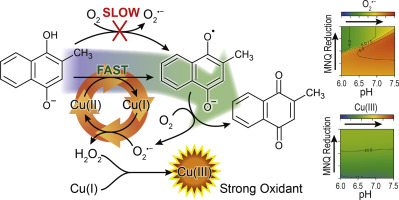
The oxidation of hydroquinones is of interest both due to the generation of reactive oxygen species (ROS) and to the implications to trace metal redox state. Menadione (MNQ), a typical toxicant quinone used extensively for studying the mechanisms underlying oxidative stress, is known to be an effective source of exogenous ROS. In this study, the kinetics and mechanism of the oxidation of menadiol (MNH2Q, the reduced form of MNQ) in the absence and presence of copper (Cu) over the pH range 6.0–7.5 was examined. The autoxidation rate increased with increasing pH and concentration of O2 and also slightly increased with increasing concentration of MNH2Q and MNQ with Cu shown to play a significant role in catalysing the oxidation of MNH2Q. A kinetic model showed that the mono-deprotonated menadiol, MNHQ−, accounted for the pH dependence of the autoxidation rate. In this proposed mechanism, both MNH2Q and MNHQ− species were oxidized quickly by Cu(II), generating menadione semiquinone (MNSQ•−) and superoxide (O2•−) and the reduced form of Cu, Cu(I). Oxygen not only facilitated the catalytic role of Cu(II) by rapidly regenerating Cu(II) but also effectively removed MSNQ•−, generating the important chain-propagating species O2•−. The model demonstrated that Cu(I) was a significant sink of O2•− resulting in the generation of H2O2 with subsequent generation of highly oxidative intermediates including Cu(III). These results provide considerable insight into the clinical significance of the biological activation and detoxification of MNQ with the kinetic model developed of use in identifying key processes in the generation of harmful oxidants in living systems.
中文翻译:

铜与甲萘醌(维生素K3)相互作用产生的氧化剂生成-一种在生命系统中金属介导的氧化剂生成的模型
氢醌的氧化不仅由于活性氧(ROS)的产生而且对痕量金属氧化还原态的影响都是令人关注的。甲萘醌(MNQ)是一种广泛用于研究氧化应激潜在机制的典型毒物醌,已知是外源性ROS的有效来源。在这项研究中,研究了在pH范围为6.0-7.5的情况下,在不存在和存在铜(Cu)的情况下,薄荷醇(MNH 2 Q,MNQ的还原形式)氧化的动力学和机理。自氧化速率随pH值和O 2浓度的增加而增加,并且随MNH 2 Q和MNQ浓度的增加而略有增加,其中Cu在催化MNH 2的氧化中起着重要作用。Q.动力学模型表明,单脱质子甲萘氢醌,MNHQ - ,占自氧化速率的pH依赖性。在这种提出的机理,二者MNH 2 Q和MNHQ -物种很快用Cu(II),生成甲萘醌半醌(MNSQ氧化- •和超氧化物(O)2 • - )和还原的Cu,铜(I)的形式。氧气不仅通过快速再生Cu(II)促进了Cu(II)的催化作用,而且还有效地除去了MSNQ •,从而生成了重要的链增长物种O 2 •。该模型表明,Cu(I)是O 2 •的重要吸收源,导致生成H2 O 2,随后生成包括Cu(III)的高氧化性中间体。这些结果为开发MNQ的动力学模型提供了对MNQ的生物激活和解毒的临床意义的重要见解,该动力学模型用于识别生命系统中有害氧化剂的产生的关键过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号