当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intramolecular Interactions of Conjugated Polymers Mimic Molecular Chaperones to Stabilize Protein–Polymer Conjugates
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1021/acs.biomac.8b00927 Stefanie L. Baker 1, 2 , Aravinda Munasinghe 3 , Hironobu Murata 2 , Ping Lin 3 , Krzysztof Matyjaszewski 4 , Coray M. Colina 3, 5 , Alan J. Russell 1, 2, 4, 6, 7
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1021/acs.biomac.8b00927 Stefanie L. Baker 1, 2 , Aravinda Munasinghe 3 , Hironobu Murata 2 , Ping Lin 3 , Krzysztof Matyjaszewski 4 , Coray M. Colina 3, 5 , Alan J. Russell 1, 2, 4, 6, 7
Affiliation
|
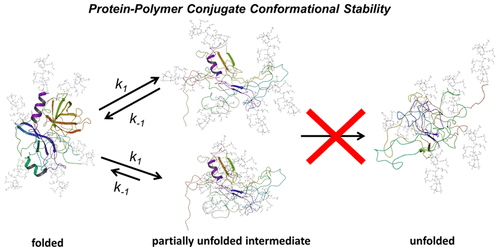
The power and elegance of protein–polymer conjugates has solved many vexing problems for society. Rational design of these complex covalent hybrids depends on a deep understanding of how polymer physicochemical properties impact the conjugate structure–function–dynamic relationships. We have generated a large family of chymotrypsin-polymer conjugates which differ in polymer length and charge, using grafting-from atom-transfer radical polymerization, to elucidate how the polymers influenced enzyme structure and function at pHs that would unfold and inactivate the enzyme. We also used molecular dynamics simulations to deepen our understanding of protein–polymer intramolecular interactions. Remarkably, the data revealed that, contrary to current thoughts on how polymers stabilize proteins, appropriately designed polymers actually stabilize partially unfolded intermediates and assist in refolding to an active conformation. Long, hydrophilic polymers minimized interfacial interactions in partially unfolded conjugates leading to increased stabilization. The design of covalently attached intramolecular biomimetic chaperones that drive protein refolding could have far reaching consequences.
中文翻译:

分子间相互作用的共轭聚合物模仿分子伴侣稳定蛋白-聚合物共轭物。
蛋白质-聚合物共轭物的强大功能和优雅解决了社会上许多棘手的问题。这些复杂的共价杂化物的合理设计取决于对聚合物理化性质如何影响共轭结构-功能-动力学关系的深刻理解。我们已经使用原子转移自由基聚合技术生成了一大类胰凝乳蛋白酶-聚合物共轭物,它们的聚合物长度和电荷不同,目的是阐明聚合物如何在pH值影响酶结构和功能的情况下将其展开和灭活,从而影响酶的结构和功能。我们还使用分子动力学模拟加深了对蛋白质-聚合物分子间相互作用的了解。值得注意的是,数据显示,与目前有关聚合物如何稳定蛋白质的想法相反,经过适当设计的聚合物实际上可以稳定部分未折叠的中间体,并有助于重新折叠成活性构象。长的亲水性聚合物可最大程度地减少部分未折叠的偶联物中的界面相互作用,从而提高稳定性。设计共价连接的分子内仿生分子伴侣驱动蛋白质的折叠可能会产生深远的影响。
更新日期:2018-08-07
中文翻译:

分子间相互作用的共轭聚合物模仿分子伴侣稳定蛋白-聚合物共轭物。
蛋白质-聚合物共轭物的强大功能和优雅解决了社会上许多棘手的问题。这些复杂的共价杂化物的合理设计取决于对聚合物理化性质如何影响共轭结构-功能-动力学关系的深刻理解。我们已经使用原子转移自由基聚合技术生成了一大类胰凝乳蛋白酶-聚合物共轭物,它们的聚合物长度和电荷不同,目的是阐明聚合物如何在pH值影响酶结构和功能的情况下将其展开和灭活,从而影响酶的结构和功能。我们还使用分子动力学模拟加深了对蛋白质-聚合物分子间相互作用的了解。值得注意的是,数据显示,与目前有关聚合物如何稳定蛋白质的想法相反,经过适当设计的聚合物实际上可以稳定部分未折叠的中间体,并有助于重新折叠成活性构象。长的亲水性聚合物可最大程度地减少部分未折叠的偶联物中的界面相互作用,从而提高稳定性。设计共价连接的分子内仿生分子伴侣驱动蛋白质的折叠可能会产生深远的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号