当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Amyloidogenicity, Cytotoxicity, and Receptor Activity of Bovine Amylin: Implications for Xenobiotic Transplantation and the Design of Nontoxic Amylin Variants
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1021/acschembio.8b00690 Rehana Akter 1 , Rebekah L. Bower 2 , Andisheh Abedini 3 , Ann Marie Schmidt 3 , Debbie L. Hay 2 , Daniel P. Raleigh 1, 4
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1021/acschembio.8b00690 Rehana Akter 1 , Rebekah L. Bower 2 , Andisheh Abedini 3 , Ann Marie Schmidt 3 , Debbie L. Hay 2 , Daniel P. Raleigh 1, 4
Affiliation
|
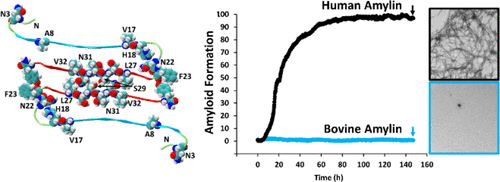
Islet amyloid formation contributes to β-cell death and dysfunction in type-2 diabetes and to the failure of islet transplants. Amylin (islet amyloid polypeptide, IAPP), a normally soluble 37 residue polypeptide hormone produced in the pancreatic β-cells, is responsible for amyloid formation in type-2 diabetes and is deficient in type-1 diabetes. Amylin normally plays an adaptive role in metabolism, and the development of nontoxic, non-amyloidogenic, bioactive variants of human amylin are of interest for use as adjuncts to insulin therapy. Naturally occurring non-amyloidogenic variants are of interest for xenobiotic transplantation and because they can provide clues toward understanding the amyloidogenicity of human amylin. The sequence of amylin is well-conserved among species, but sequence differences strongly correlate with in vitro amyloidogenicity and with islet amyloid formation in vivo. Bovine amylin differs from the human peptide at 10 positions and is one of the most divergent among known amylin sequences. We show that bovine amylin oligomerizes but is not toxic to cultured β-cells and that it is considerably less amyloidogenic than the human polypeptide and is only a low-potency agonist at human amylin-responsive receptors. The bovine sequence contains several nonconservative substitutions relative to human amylin, including His to Pro, Ser to Pro, and Asn to Lys replacements. The effect of these substitutions is analyzed in the context of wild-type human amylin; the results provide insight into their role in receptor activation, the mode of assembly of human amylin, and the design of soluble amylin analogues.
中文翻译:

牛淀粉样蛋白的淀粉样变性,细胞毒性和受体活性:对异种生物移植和无毒淀粉样蛋白变体的设计的影响。
胰岛淀粉样蛋白的形成导致2型糖尿病的β细胞死亡和功能障碍,并导致胰岛移植失败。胰岛淀粉样多肽(胰岛淀粉样多肽,IAPP)是在胰腺β细胞中产生的通常可溶的37个残基的多肽激素,可导致2型糖尿病的淀粉样蛋白形成,并缺乏1型糖尿病。胰岛淀粉样多肽通常在新陈代谢中发挥适应性作用,而人类胰岛淀粉样多肽的无毒,非淀粉样生成,生物活性变体的发展作为胰岛素治疗的辅助手段是令人感兴趣的。天然存在的非淀粉样变体对于异种生物移植很重要,因为它们可以为理解人胰岛淀粉样蛋白的淀粉样变性提供线索。胰岛淀粉样多肽的序列在物种之间是保守的,但是序列差异与体外具有淀粉样变性,并在体内具有胰岛淀粉样形成。牛胰岛淀粉样多肽在十个位置与人肽不同,并且是已知胰岛淀粉样多肽序列中差异最大的多肽之一。我们表明,牛胰岛淀粉样多肽低聚,但对培养的β细胞无毒,它的淀粉样变性比人多肽低得多,并且是对人胰岛淀粉样蛋白应答受体的低效激动剂。相对于人胰岛淀粉样多肽,牛序列包含多个非保守取代,包括His取代Pro,Ser取代Pro和Asn取代Lys。在野生型人胰岛淀粉样多肽的背景下分析了这些取代的作用。该结果提供了对它们在受体激活中的作用,人类糊精组装模式以及可溶性糊精类似物设计的见解。
更新日期:2018-08-07
中文翻译:

牛淀粉样蛋白的淀粉样变性,细胞毒性和受体活性:对异种生物移植和无毒淀粉样蛋白变体的设计的影响。
胰岛淀粉样蛋白的形成导致2型糖尿病的β细胞死亡和功能障碍,并导致胰岛移植失败。胰岛淀粉样多肽(胰岛淀粉样多肽,IAPP)是在胰腺β细胞中产生的通常可溶的37个残基的多肽激素,可导致2型糖尿病的淀粉样蛋白形成,并缺乏1型糖尿病。胰岛淀粉样多肽通常在新陈代谢中发挥适应性作用,而人类胰岛淀粉样多肽的无毒,非淀粉样生成,生物活性变体的发展作为胰岛素治疗的辅助手段是令人感兴趣的。天然存在的非淀粉样变体对于异种生物移植很重要,因为它们可以为理解人胰岛淀粉样蛋白的淀粉样变性提供线索。胰岛淀粉样多肽的序列在物种之间是保守的,但是序列差异与体外具有淀粉样变性,并在体内具有胰岛淀粉样形成。牛胰岛淀粉样多肽在十个位置与人肽不同,并且是已知胰岛淀粉样多肽序列中差异最大的多肽之一。我们表明,牛胰岛淀粉样多肽低聚,但对培养的β细胞无毒,它的淀粉样变性比人多肽低得多,并且是对人胰岛淀粉样蛋白应答受体的低效激动剂。相对于人胰岛淀粉样多肽,牛序列包含多个非保守取代,包括His取代Pro,Ser取代Pro和Asn取代Lys。在野生型人胰岛淀粉样多肽的背景下分析了这些取代的作用。该结果提供了对它们在受体激活中的作用,人类糊精组装模式以及可溶性糊精类似物设计的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号