当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand and solvent control of selectivity in the C–H activation of a pyridylimine-substituted 1-naphthalene; a combined synthetic and computational study†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1039/c8dt02565g Rena Simayi 1, 2, 3, 4 , Simone M. Gillbard 4, 5, 6, 7 , Warren B. Cross 4, 5, 6, 7 , Eric G. Hope 1, 2, 3, 4 , Kuldip Singh 1, 2, 3, 4 , Gregory A. Solan 1, 2, 3, 4
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-07 00:00:00 , DOI: 10.1039/c8dt02565g Rena Simayi 1, 2, 3, 4 , Simone M. Gillbard 4, 5, 6, 7 , Warren B. Cross 4, 5, 6, 7 , Eric G. Hope 1, 2, 3, 4 , Kuldip Singh 1, 2, 3, 4 , Gregory A. Solan 1, 2, 3, 4
Affiliation
|
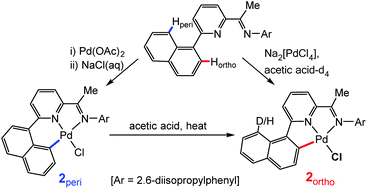
The pyridylimine-substituted 1-naphthalenes, 2-(1-C10H7)-6-{CR![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(2,6-i-Pr2C6H3)}C5H3N (R = Me HLMe, H HLH), react with Na2[PdCl4] in acetic acid at elevated temperature to afford either ortho-C–Hnaphthyl activated (LMe)PdCl (2ortho) or the unactivated adduct (HLH)PdCl2 (1b). Alternatively, 1b and its ketimine analogue (HLMe)PdCl2 (1a), can be prepared by treating (MeCN)2PdCl2 with either HLMe or HLH in chloroform at room temperature. Regio-selective ortho-C–H activation to form 2ortho can also be initiated by the thermolysis of 1a in acetic acid, while no reaction occurs under similar conditions with 1b. Interestingly, the C–H activation of HLMe to give 2ortho is found to be reversible with 100% deuteration of the peri-site occurring on reacting Na2[PdCl4] with HLMe in acetic acid-d4. By contrast, heating 1a in toluene gives a 55 : 45 mixture of 2ortho and its peri-activated isomer 2peri. Pure 2peri can, however, be obtained either from (LMe)PdOAc (3peri) by OAc/Cl exchange or by the sequential reactions of 1a with firstly silver acetate then with aqueous sodium chloride. Intriguingly, a peri to ortho interconversion occurs on heating 2peri in acetic acid to give 2ortho. DFT calculations have been used to investigate the C–H activation steps and it is found that in acetic acid ortho-C–H activation is kinetically and thermodynamically favoured but peri-CH activation is kinetically accessible (ΔΔG‡ = 2.4 kcal mol−1). By contrast in toluene, the reaction appears to be irreversible with the difference in barrier height for ortho- and peri-C–H activation being very small within the error of the method (ΔΔG‡ = 0.7 kcal mol−1), findings that are in agreement with the empirically observed product distribution for 2ortho and 2peri. Single crystal X-ray structures are reported for 1a, 1b, 2ortho and 2peri.
N(2,6-i-Pr2C6H3)}C5H3N (R = Me HLMe, H HLH), react with Na2[PdCl4] in acetic acid at elevated temperature to afford either ortho-C–Hnaphthyl activated (LMe)PdCl (2ortho) or the unactivated adduct (HLH)PdCl2 (1b). Alternatively, 1b and its ketimine analogue (HLMe)PdCl2 (1a), can be prepared by treating (MeCN)2PdCl2 with either HLMe or HLH in chloroform at room temperature. Regio-selective ortho-C–H activation to form 2ortho can also be initiated by the thermolysis of 1a in acetic acid, while no reaction occurs under similar conditions with 1b. Interestingly, the C–H activation of HLMe to give 2ortho is found to be reversible with 100% deuteration of the peri-site occurring on reacting Na2[PdCl4] with HLMe in acetic acid-d4. By contrast, heating 1a in toluene gives a 55 : 45 mixture of 2ortho and its peri-activated isomer 2peri. Pure 2peri can, however, be obtained either from (LMe)PdOAc (3peri) by OAc/Cl exchange or by the sequential reactions of 1a with firstly silver acetate then with aqueous sodium chloride. Intriguingly, a peri to ortho interconversion occurs on heating 2peri in acetic acid to give 2ortho. DFT calculations have been used to investigate the C–H activation steps and it is found that in acetic acid ortho-C–H activation is kinetically and thermodynamically favoured but peri-CH activation is kinetically accessible (ΔΔG‡ = 2.4 kcal mol−1). By contrast in toluene, the reaction appears to be irreversible with the difference in barrier height for ortho- and peri-C–H activation being very small within the error of the method (ΔΔG‡ = 0.7 kcal mol−1), findings that are in agreement with the empirically observed product distribution for 2ortho and 2peri. Single crystal X-ray structures are reported for 1a, 1b, 2ortho and 2peri.
中文翻译:

吡啶和嘧啶取代的1-萘在CH活化中的选择性配体和溶剂控制;综合研究与计算研究†
吡啶基吡啶取代的1-萘2-(1-C 10 H 7)-6- {CR![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) N(2,6-i-Pr 2 C 6 H 3)} C 5 H 3 N(R = Me H L Me(HH L H)与乙酸中的Na 2 [PdCl 4 ]在升高的温度下反应,得到邻位C–H萘基活化的(L Me)PdCl(邻位2)或未活化的加合物(H L H)PdCl 2(1b)。或者,1b及其酮亚胺类似物(H L Me)PdCl 2(1a)可以通过在室温下在氯仿中用H L Me或H L H处理(MeCN)2 PdCl 2来制备。1a在乙酸中的热解也可引发区域选择性邻-C–H活化形成2个邻,而在类似条件下与1b不会发生任何反应。有趣的是,H L Me的C–H活化得到2个邻位被发现是可逆的100%氘围上的Na反应发生-site 2 [的PdCl 4 ]用H大号我在乙酸- d 4。相反,加热1A在甲苯给出了一个55:45混合物2邻位和它的周围激活的异构体2点周围。纯2围可以,但是,可以从任一(获得大号我)PdOAc(3围)由OAC / CL交换或通过的顺序反应1A先用乙酸银,再用氯化钠水溶液。有趣的是,一迫到邻位相互发生在加热2围在乙酸,得到2邻位。DFT计算已经被用于研究C-H活化步骤和发现在乙酸邻-C-H活化动力学和热力学上有利,但围-CH活化是动力学上可访问的(ΔΔ ģ ‡ = 2.4千卡摩尔-1)。通过在甲苯相比之下,反应似乎是不可逆的,在对势垒高度的差邻-和围-C-H活化是非常小的方法的误差(ΔΔ内ģ ‡ = 0.7千卡摩尔-1),发现,即在协议与用于经验观察产物分布2点的邻位和2周围。报告了1a,1b,2个邻位和2个peri的单晶X射线结构。
N(2,6-i-Pr 2 C 6 H 3)} C 5 H 3 N(R = Me H L Me(HH L H)与乙酸中的Na 2 [PdCl 4 ]在升高的温度下反应,得到邻位C–H萘基活化的(L Me)PdCl(邻位2)或未活化的加合物(H L H)PdCl 2(1b)。或者,1b及其酮亚胺类似物(H L Me)PdCl 2(1a)可以通过在室温下在氯仿中用H L Me或H L H处理(MeCN)2 PdCl 2来制备。1a在乙酸中的热解也可引发区域选择性邻-C–H活化形成2个邻,而在类似条件下与1b不会发生任何反应。有趣的是,H L Me的C–H活化得到2个邻位被发现是可逆的100%氘围上的Na反应发生-site 2 [的PdCl 4 ]用H大号我在乙酸- d 4。相反,加热1A在甲苯给出了一个55:45混合物2邻位和它的周围激活的异构体2点周围。纯2围可以,但是,可以从任一(获得大号我)PdOAc(3围)由OAC / CL交换或通过的顺序反应1A先用乙酸银,再用氯化钠水溶液。有趣的是,一迫到邻位相互发生在加热2围在乙酸,得到2邻位。DFT计算已经被用于研究C-H活化步骤和发现在乙酸邻-C-H活化动力学和热力学上有利,但围-CH活化是动力学上可访问的(ΔΔ ģ ‡ = 2.4千卡摩尔-1)。通过在甲苯相比之下,反应似乎是不可逆的,在对势垒高度的差邻-和围-C-H活化是非常小的方法的误差(ΔΔ内ģ ‡ = 0.7千卡摩尔-1),发现,即在协议与用于经验观察产物分布2点的邻位和2周围。报告了1a,1b,2个邻位和2个peri的单晶X射线结构。
更新日期:2018-08-07
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(2,6-i-Pr2C6H3)}C5H3N (R = Me HLMe, H HLH), react with Na2[PdCl4] in acetic acid at elevated temperature to afford either ortho-C–Hnaphthyl activated (LMe)PdCl (2ortho) or the unactivated adduct (HLH)PdCl2 (1b). Alternatively, 1b and its ketimine analogue (HLMe)PdCl2 (1a), can be prepared by treating (MeCN)2PdCl2 with either HLMe or HLH in chloroform at room temperature. Regio-selective ortho-C–H activation to form 2ortho can also be initiated by the thermolysis of 1a in acetic acid, while no reaction occurs under similar conditions with 1b. Interestingly, the C–H activation of HLMe to give 2ortho is found to be reversible with 100% deuteration of the peri-site occurring on reacting Na2[PdCl4] with HLMe in acetic acid-d4. By contrast, heating 1a in toluene gives a 55 : 45 mixture of 2ortho and its peri-activated isomer 2peri. Pure 2peri can, however, be obtained either from (LMe)PdOAc (3peri) by OAc/Cl exchange or by the sequential reactions of 1a with firstly silver acetate then with aqueous sodium chloride. Intriguingly, a peri to ortho interconversion occurs on heating 2peri in acetic acid to give 2ortho. DFT calculations have been used to investigate the C–H activation steps and it is found that in acetic acid ortho-C–H activation is kinetically and thermodynamically favoured but peri-CH activation is kinetically accessible (ΔΔG‡ = 2.4 kcal mol−1). By contrast in toluene, the reaction appears to be irreversible with the difference in barrier height for ortho- and peri-C–H activation being very small within the error of the method (ΔΔG‡ = 0.7 kcal mol−1), findings that are in agreement with the empirically observed product distribution for 2ortho and 2peri. Single crystal X-ray structures are reported for 1a, 1b, 2ortho and 2peri.
N(2,6-i-Pr2C6H3)}C5H3N (R = Me HLMe, H HLH), react with Na2[PdCl4] in acetic acid at elevated temperature to afford either ortho-C–Hnaphthyl activated (LMe)PdCl (2ortho) or the unactivated adduct (HLH)PdCl2 (1b). Alternatively, 1b and its ketimine analogue (HLMe)PdCl2 (1a), can be prepared by treating (MeCN)2PdCl2 with either HLMe or HLH in chloroform at room temperature. Regio-selective ortho-C–H activation to form 2ortho can also be initiated by the thermolysis of 1a in acetic acid, while no reaction occurs under similar conditions with 1b. Interestingly, the C–H activation of HLMe to give 2ortho is found to be reversible with 100% deuteration of the peri-site occurring on reacting Na2[PdCl4] with HLMe in acetic acid-d4. By contrast, heating 1a in toluene gives a 55 : 45 mixture of 2ortho and its peri-activated isomer 2peri. Pure 2peri can, however, be obtained either from (LMe)PdOAc (3peri) by OAc/Cl exchange or by the sequential reactions of 1a with firstly silver acetate then with aqueous sodium chloride. Intriguingly, a peri to ortho interconversion occurs on heating 2peri in acetic acid to give 2ortho. DFT calculations have been used to investigate the C–H activation steps and it is found that in acetic acid ortho-C–H activation is kinetically and thermodynamically favoured but peri-CH activation is kinetically accessible (ΔΔG‡ = 2.4 kcal mol−1). By contrast in toluene, the reaction appears to be irreversible with the difference in barrier height for ortho- and peri-C–H activation being very small within the error of the method (ΔΔG‡ = 0.7 kcal mol−1), findings that are in agreement with the empirically observed product distribution for 2ortho and 2peri. Single crystal X-ray structures are reported for 1a, 1b, 2ortho and 2peri.
中文翻译:

吡啶和嘧啶取代的1-萘在CH活化中的选择性配体和溶剂控制;综合研究与计算研究†
吡啶基吡啶取代的1-萘2-(1-C 10 H 7)-6- {CR
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) N(2,6-i-Pr 2 C 6 H 3)} C 5 H 3 N(R = Me H L Me(HH L H)与乙酸中的Na 2 [PdCl 4 ]在升高的温度下反应,得到邻位C–H萘基活化的(L Me)PdCl(邻位2)或未活化的加合物(H L H)PdCl 2(1b)。或者,1b及其酮亚胺类似物(H L Me)PdCl 2(1a)可以通过在室温下在氯仿中用H L Me或H L H处理(MeCN)2 PdCl 2来制备。1a在乙酸中的热解也可引发区域选择性邻-C–H活化形成2个邻,而在类似条件下与1b不会发生任何反应。有趣的是,H L Me的C–H活化得到2个邻位被发现是可逆的100%氘围上的Na反应发生-site 2 [的PdCl 4 ]用H大号我在乙酸- d 4。相反,加热1A在甲苯给出了一个55:45混合物2邻位和它的周围激活的异构体2点周围。纯2围可以,但是,可以从任一(获得大号我)PdOAc(3围)由OAC / CL交换或通过的顺序反应1A先用乙酸银,再用氯化钠水溶液。有趣的是,一迫到邻位相互发生在加热2围在乙酸,得到2邻位。DFT计算已经被用于研究C-H活化步骤和发现在乙酸邻-C-H活化动力学和热力学上有利,但围-CH活化是动力学上可访问的(ΔΔ ģ ‡ = 2.4千卡摩尔-1)。通过在甲苯相比之下,反应似乎是不可逆的,在对势垒高度的差邻-和围-C-H活化是非常小的方法的误差(ΔΔ内ģ ‡ = 0.7千卡摩尔-1),发现,即在协议与用于经验观察产物分布2点的邻位和2周围。报告了1a,1b,2个邻位和2个peri的单晶X射线结构。
N(2,6-i-Pr 2 C 6 H 3)} C 5 H 3 N(R = Me H L Me(HH L H)与乙酸中的Na 2 [PdCl 4 ]在升高的温度下反应,得到邻位C–H萘基活化的(L Me)PdCl(邻位2)或未活化的加合物(H L H)PdCl 2(1b)。或者,1b及其酮亚胺类似物(H L Me)PdCl 2(1a)可以通过在室温下在氯仿中用H L Me或H L H处理(MeCN)2 PdCl 2来制备。1a在乙酸中的热解也可引发区域选择性邻-C–H活化形成2个邻,而在类似条件下与1b不会发生任何反应。有趣的是,H L Me的C–H活化得到2个邻位被发现是可逆的100%氘围上的Na反应发生-site 2 [的PdCl 4 ]用H大号我在乙酸- d 4。相反,加热1A在甲苯给出了一个55:45混合物2邻位和它的周围激活的异构体2点周围。纯2围可以,但是,可以从任一(获得大号我)PdOAc(3围)由OAC / CL交换或通过的顺序反应1A先用乙酸银,再用氯化钠水溶液。有趣的是,一迫到邻位相互发生在加热2围在乙酸,得到2邻位。DFT计算已经被用于研究C-H活化步骤和发现在乙酸邻-C-H活化动力学和热力学上有利,但围-CH活化是动力学上可访问的(ΔΔ ģ ‡ = 2.4千卡摩尔-1)。通过在甲苯相比之下,反应似乎是不可逆的,在对势垒高度的差邻-和围-C-H活化是非常小的方法的误差(ΔΔ内ģ ‡ = 0.7千卡摩尔-1),发现,即在协议与用于经验观察产物分布2点的邻位和2周围。报告了1a,1b,2个邻位和2个peri的单晶X射线结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号