当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteomic Toolbox To Standardize the Separation of Extracellular Vesicles and Lipoprotein Particles.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-08-16 , DOI: 10.1021/acs.jproteome.8b00225 Tingting Wang 1, 2 , Illarion V Turko 1, 2
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-08-16 , DOI: 10.1021/acs.jproteome.8b00225 Tingting Wang 1, 2 , Illarion V Turko 1, 2
Affiliation
|
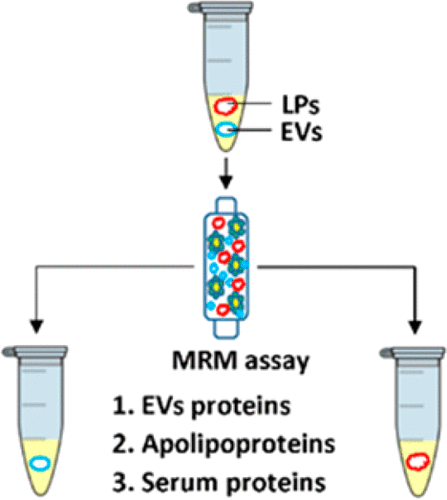
Circulating in blood, extracellular vesicles (EVs) and lipoprotein particles (LPs) have diagnostic and prognostic value. To unambiguously define their functions, separation protocols need to be developed. However, because of their similar size and density, traditional approaches to separate EVs and LPs often fail to provide the required resolution. Further development and standardization of affinity-based protocols is necessary, and a quantitative method is needed to assess the efficiency of LP depletion from EV samples. In the present study, we propose the simultaneous quantification of three groups of proteins by mass spectrometry as a toolbox to evaluate prospective separation protocols. We generated 15N-labeled internal standards for quantification of (i) EV-specific proteins, (ii) all classes and subclasses of apolipoproteins constituting LPs, and (iii) several major serum proteins. These standards were then used in multiple reaction monitoring assay to evaluate the performance of size-exclusion chromatography, heparin-Sepharose, lipopolysaccharide-Sepharose, (2-hydroxypropyl)-β-cyclodextrin-Sepharose, and concanavalin A-Sepharose in separating serum EVs and LPs. The efficiency of a resin to separate EVs from non-EV substances could be jeopardized by simultaneous EV aggregation. Therefore, dynamic light scattering analysis was used in this study in addition to the proteomic toolbox when making a recommendation to use particular resin for EV isolation. On the basis of our measurements, we concluded that none of the individual separation protocols used in this study resulted in LP-free EVs, and the combination of two protocols may be complex due to low EV yield. Overall, this further points to the importance of proposed proteomic toolbox for the future evaluation of EV separation protocols.
中文翻译:

蛋白质组学工具箱,用于标准化细胞外囊泡和脂蛋白颗粒的分离。
在血液中循环,细胞外囊泡(EV)和脂蛋白颗粒(LP)具有诊断和预后价值。为了明确定义其功能,需要开发分离协议。然而,由于其相似的尺寸和密度,分离电动汽车和电动汽车的传统方法通常无法提供所需的分辨率。基于亲和力的协议的进一步开发和标准化是必要的,并且需要一种定量方法来评估EV样品中LP消耗的效率。在本研究中,我们建议通过质谱法同时定量三组蛋白质,作为评估预期分离方案的工具箱。我们产生了15N标记的内标,用于定量(i)EV特异性蛋白,(ii)构成LP的载脂蛋白的所有类别和亚类,以及(iii)几种主要的血清蛋白。然后将这些标准品用于多重反应监测分析中,以评估尺寸排阻色谱法,肝素-琼脂糖,脂多糖-琼脂糖,(2-羟丙基)-β-环糊精-琼脂糖和伴刀豆球蛋白A-琼脂糖在分离血清EV和LP。同时进行EV聚合可能会损害树脂将EV与非EV物质分离的效率。因此,在建议使用特定树脂进行EV隔离的建议时,除蛋白质组学工具箱外,本研究还使用了动态光散射分析。根据我们的测量,我们得出的结论是,本研究中使用的单个分离方案均未产生无LP电动汽车,并且由于电动汽车产量低,两种方案的组合可能很复杂。总体而言,这进一步指出了拟议的蛋白质组学工具箱对于EV分离方案的未来评估的重要性。
更新日期:2018-08-17
中文翻译:

蛋白质组学工具箱,用于标准化细胞外囊泡和脂蛋白颗粒的分离。
在血液中循环,细胞外囊泡(EV)和脂蛋白颗粒(LP)具有诊断和预后价值。为了明确定义其功能,需要开发分离协议。然而,由于其相似的尺寸和密度,分离电动汽车和电动汽车的传统方法通常无法提供所需的分辨率。基于亲和力的协议的进一步开发和标准化是必要的,并且需要一种定量方法来评估EV样品中LP消耗的效率。在本研究中,我们建议通过质谱法同时定量三组蛋白质,作为评估预期分离方案的工具箱。我们产生了15N标记的内标,用于定量(i)EV特异性蛋白,(ii)构成LP的载脂蛋白的所有类别和亚类,以及(iii)几种主要的血清蛋白。然后将这些标准品用于多重反应监测分析中,以评估尺寸排阻色谱法,肝素-琼脂糖,脂多糖-琼脂糖,(2-羟丙基)-β-环糊精-琼脂糖和伴刀豆球蛋白A-琼脂糖在分离血清EV和LP。同时进行EV聚合可能会损害树脂将EV与非EV物质分离的效率。因此,在建议使用特定树脂进行EV隔离的建议时,除蛋白质组学工具箱外,本研究还使用了动态光散射分析。根据我们的测量,我们得出的结论是,本研究中使用的单个分离方案均未产生无LP电动汽车,并且由于电动汽车产量低,两种方案的组合可能很复杂。总体而言,这进一步指出了拟议的蛋白质组学工具箱对于EV分离方案的未来评估的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号