当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical Considerations for the Formation of Acyl Imidazolides from Carboxylic Acids and N,N′-Carbonyldiimidazole: The Role of Acid Catalysis
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acs.oprd.8b00121 Kenneth M. Engstrom 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acs.oprd.8b00121 Kenneth M. Engstrom 1
Affiliation
|
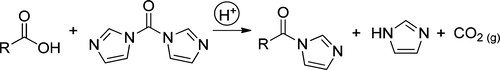
The conversion of carboxylic acids to acyl imidazolides using N,N′-carbonyldiimidazole (CDI) is hampered by the presence of alkali salts of the carboxylic acid, resulting in incomplete reactions, which cannot be driven to completion with excess CDI. Addition of an exogenous proton source reverses this effect. Sparging of the reaction mixture with carbon dioxide is also effective, presumably due to the formation of the carbamic acid of imidazole, which acts as a proton source. These results suggest that acyl imidazolide formation is subject to acid catalysis. The acceleration of acyl imidazolide formation using triflic acid or imidazolium triflate, as catalyst, is demonstrated.
中文翻译:

从羧酸和N,N'-羰基二咪唑形成酰基咪唑化物的实际考虑:酸催化的作用
羧酸碱盐的存在阻碍了使用N,N'-羰基二咪唑(CDI)的羧酸向酰基咪唑啉的转化,从而导致反应不完全,无法通过过量的CDI驱使反应完成。添加外源质子源可以逆转这种影响。用二氧化碳喷射反应混合物也是有效的,大概是由于形成了作为质子源的咪唑氨基甲酸酯的形成。这些结果表明酰基咪唑啉化物的形成受酸催化。证明了使用三氟甲磺酸或三氟甲磺酸咪唑鎓盐作为催化剂促进酰基咪唑啉化物的形成。
更新日期:2018-08-06
中文翻译:

从羧酸和N,N'-羰基二咪唑形成酰基咪唑化物的实际考虑:酸催化的作用
羧酸碱盐的存在阻碍了使用N,N'-羰基二咪唑(CDI)的羧酸向酰基咪唑啉的转化,从而导致反应不完全,无法通过过量的CDI驱使反应完成。添加外源质子源可以逆转这种影响。用二氧化碳喷射反应混合物也是有效的,大概是由于形成了作为质子源的咪唑氨基甲酸酯的形成。这些结果表明酰基咪唑啉化物的形成受酸催化。证明了使用三氟甲磺酸或三氟甲磺酸咪唑鎓盐作为催化剂促进酰基咪唑啉化物的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号