当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fate of Liposomes in the Presence of Phospholipase C and D: From Atomic to Supramolecular Lipid Arrangement
ACS Central Science ( IF 12.7 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acscentsci.8b00286 Margaret N Holme 1, 2, 2, 3 , M Harunur Rashid 4 , Michael R Thomas 1, 2, 2 , Hanna M G Barriga 3 , Karla Luise Herpoldt 1, 2, 2 , Richard K Heenan 5 , Cécile A Dreiss 6 , José Leobardo Bañuelos 5, 7 , Hai-Nan Xie 1, 2, 2 , Irene Yarovsky 4 , Molly M Stevens 1, 2, 2, 3
ACS Central Science ( IF 12.7 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acscentsci.8b00286 Margaret N Holme 1, 2, 2, 3 , M Harunur Rashid 4 , Michael R Thomas 1, 2, 2 , Hanna M G Barriga 3 , Karla Luise Herpoldt 1, 2, 2 , Richard K Heenan 5 , Cécile A Dreiss 6 , José Leobardo Bañuelos 5, 7 , Hai-Nan Xie 1, 2, 2 , Irene Yarovsky 4 , Molly M Stevens 1, 2, 2, 3
Affiliation
|
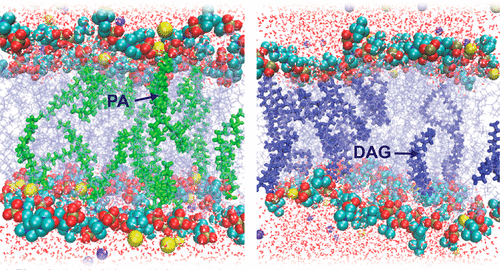
Understanding the origins of lipid membrane bilayer rearrangement in response to external stimuli is an essential component of cell biology and the bottom-up design of liposomes for biomedical applications. The enzymes phospholipase C and D (PLC and PLD) both cleave the phosphorus–oxygen bonds of phosphate esters in phosphatidylcholine (PC) lipids. The atomic position of this hydrolysis reaction has huge implications for the stability of PC-containing self-assembled structures, such as the cell wall and lipid-based vesicle drug delivery vectors. While PLC converts PC to diacylglycerol (DAG), the interaction of PC with PLD produces phosphatidic acid (PA). Here we present a combination of small-angle scattering data and all-atom molecular dynamics simulations, providing insights into the effects of atomic-scale reorganization on the supramolecular assembly of PC membrane bilayers upon enzyme-mediated incorporation of DAG or PA. We observed that PC liposomes completely disintegrate in the presence of PLC, as conversion of PC to DAG progresses. At lower concentrations, DAG molecules within fluid PC bilayers form hydrogen bonds with backbone carbonyl oxygens in neighboring PC molecules and burrow into the hydrophobic region. This leads initially to membrane thinning followed by a swelling of the lamellar phase with increased DAG. At higher DAG concentrations, localized membrane tension causes a change in lipid phase from lamellar to the hexagonal and micellar cubic phases. Molecular dynamics simulations show that this destabilization is also caused in part by the decreased ability of DAG-containing PC membranes to coordinate sodium ions. Conversely, PLD-treated PC liposomes remain stable up to extremely high conversions to PA. Here, the negatively charged PA headgroup attracts significant amounts of sodium ions from the bulk solution to the membrane surface, leading to a swelling of the coordinated water layer. These findings are a vital step toward a fundamental understanding of the degradation behavior of PC lipid membranes in the presence of these clinically relevant enzymes, and toward the rational design of diagnostic and drug delivery technologies for phospholipase-dysregulation-based diseases.
中文翻译:

磷脂酶 C 和 D 存在下脂质体的命运:从原子到超分子脂质排列
了解响应外部刺激的脂质膜双层重排的起源是细胞生物学和自下而上设计用于生物医学应用的脂质体的重要组成部分。磷脂酶 C 和 D(PLC 和 PLD)都切割磷脂酰胆碱(PC)脂质中磷酸酯的磷氧键。这种水解反应的原子位置对含有 PC 的自组装结构的稳定性具有巨大的影响,例如细胞壁和基于脂质的囊泡药物递送载体。虽然 PLC 将 PC 转化为二酰基甘油 (DAG),但 PC 与 PLD 的相互作用会产生磷脂酸 (PA)。在这里,我们结合了小角度散射数据和全原子分子动力学模拟,通过酶介导的 DAG 或 PA 掺入,深入了解原子级重组对 PC 膜双层超分子组装的影响。我们观察到 PC 脂质体在 PLC 存在下完全分解,随着 PC 转化为 DAG 的进展。在较低浓度下,流体 PC 双层内的 DAG 分子与相邻 PC 分子中的骨架羰基氧形成氢键并钻入疏水区域。这首先导致膜变薄,然后随着 DAG 的增加层状相膨胀。在较高的 DAG 浓度下,局部膜张力导致脂质相从层状变为六角形和胶束立方相。分子动力学模拟表明,这种不稳定的部分原因是含 DAG 的 PC 膜协调钠离子的能力下降。相反,PLD 处理的 PC 脂质体在转化为 PA 的情况下保持稳定。在这里,带负电荷的 PA 头基将大量钠离子从本体溶液吸引到膜表面,导致配位水层膨胀。这些发现是朝着基本了解在这些临床相关酶存在下 PC 脂质膜的降解行为以及合理设计基于磷脂酶失调的疾病的诊断和药物递送技术迈出的重要一步。PLD 处理的 PC 脂质体在转化为 PA 的情况下保持稳定。在这里,带负电荷的 PA 头基将大量钠离子从本体溶液吸引到膜表面,导致配位水层膨胀。这些发现是朝着基本了解在这些临床相关酶存在下 PC 脂质膜的降解行为以及合理设计基于磷脂酶失调的疾病的诊断和药物递送技术迈出的重要一步。PLD 处理的 PC 脂质体在转化为 PA 的情况下保持稳定。在这里,带负电荷的 PA 头基将大量钠离子从本体溶液吸引到膜表面,导致配位水层膨胀。这些发现是朝着基本了解在这些临床相关酶存在下 PC 脂质膜的降解行为以及合理设计基于磷脂酶失调的疾病的诊断和药物递送技术迈出的重要一步。
更新日期:2018-08-06
中文翻译:

磷脂酶 C 和 D 存在下脂质体的命运:从原子到超分子脂质排列
了解响应外部刺激的脂质膜双层重排的起源是细胞生物学和自下而上设计用于生物医学应用的脂质体的重要组成部分。磷脂酶 C 和 D(PLC 和 PLD)都切割磷脂酰胆碱(PC)脂质中磷酸酯的磷氧键。这种水解反应的原子位置对含有 PC 的自组装结构的稳定性具有巨大的影响,例如细胞壁和基于脂质的囊泡药物递送载体。虽然 PLC 将 PC 转化为二酰基甘油 (DAG),但 PC 与 PLD 的相互作用会产生磷脂酸 (PA)。在这里,我们结合了小角度散射数据和全原子分子动力学模拟,通过酶介导的 DAG 或 PA 掺入,深入了解原子级重组对 PC 膜双层超分子组装的影响。我们观察到 PC 脂质体在 PLC 存在下完全分解,随着 PC 转化为 DAG 的进展。在较低浓度下,流体 PC 双层内的 DAG 分子与相邻 PC 分子中的骨架羰基氧形成氢键并钻入疏水区域。这首先导致膜变薄,然后随着 DAG 的增加层状相膨胀。在较高的 DAG 浓度下,局部膜张力导致脂质相从层状变为六角形和胶束立方相。分子动力学模拟表明,这种不稳定的部分原因是含 DAG 的 PC 膜协调钠离子的能力下降。相反,PLD 处理的 PC 脂质体在转化为 PA 的情况下保持稳定。在这里,带负电荷的 PA 头基将大量钠离子从本体溶液吸引到膜表面,导致配位水层膨胀。这些发现是朝着基本了解在这些临床相关酶存在下 PC 脂质膜的降解行为以及合理设计基于磷脂酶失调的疾病的诊断和药物递送技术迈出的重要一步。PLD 处理的 PC 脂质体在转化为 PA 的情况下保持稳定。在这里,带负电荷的 PA 头基将大量钠离子从本体溶液吸引到膜表面,导致配位水层膨胀。这些发现是朝着基本了解在这些临床相关酶存在下 PC 脂质膜的降解行为以及合理设计基于磷脂酶失调的疾病的诊断和药物递送技术迈出的重要一步。PLD 处理的 PC 脂质体在转化为 PA 的情况下保持稳定。在这里,带负电荷的 PA 头基将大量钠离子从本体溶液吸引到膜表面,导致配位水层膨胀。这些发现是朝着基本了解在这些临床相关酶存在下 PC 脂质膜的降解行为以及合理设计基于磷脂酶失调的疾病的诊断和药物递送技术迈出的重要一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号