当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric “Acetylenic” [3+2] Cycloaddition of Nitrones Catalyzed by Cationic Chiral PdII Lewis Acid
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-08-29 , DOI: 10.1002/asia.201801016 Kazuya Honda 1 , Koichi Mikami 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-08-29 , DOI: 10.1002/asia.201801016 Kazuya Honda 1 , Koichi Mikami 1
Affiliation
|
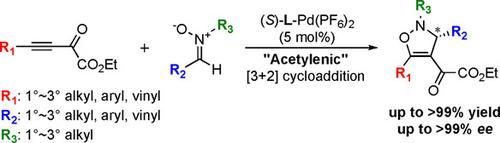
Highly enantioselective [3+2] cycloaddition of ynones and nitrones has been developed. Very bulky ligand, DTBM‐SEGPHOS, was used for an effective asymmetric induction over distal reaction centers on the linear ynone dipolarophile and for prevention of PdII catalyst deactivation by coordination of the nitrones. The reaction has wide scope of substrates in both ynones and nitrones.
中文翻译:

阳离子手性PdII Lewis酸催化的硝基的不对称“乙炔” [3 + 2]环加成反应
已经开发出对映体和硝酮的高度对映选择性的[3 + 2]环加成反应。很大体积的配体DTBM-SEGPHOS用于在线性乙炔双极性亲和体的远端反应中心进行有效的不对称诱导,并通过硝酮的配位防止Pd II催化剂失活。该反应在炔酮和硝酮中具有广泛的底物范围。
更新日期:2018-08-29
中文翻译:

阳离子手性PdII Lewis酸催化的硝基的不对称“乙炔” [3 + 2]环加成反应
已经开发出对映体和硝酮的高度对映选择性的[3 + 2]环加成反应。很大体积的配体DTBM-SEGPHOS用于在线性乙炔双极性亲和体的远端反应中心进行有效的不对称诱导,并通过硝酮的配位防止Pd II催化剂失活。该反应在炔酮和硝酮中具有广泛的底物范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号