当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and antiviral evaluation of novel hydrazone‐substituted thiophene[3,2‐d]pyrimidine derivatives as potent human immunodeficiency virus‐1 inhibitors
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-08-26 , DOI: 10.1111/cbdd.13373 Zhao Wang 1 , Dongwei Kang 1 , Meng Chen 2 , Gaochan Wu 1 , Da Feng 1 , Tong Zhao 1 , Zhongxia Zhou 1 , Zhipeng Huo 1 , Lanlan Jing 1 , Xiaofang Zuo 1 , Dirk Daelemans 3 , Erik De Clercq 3 , Christophe Pannecouque 3 , Peng Zhan 1 , Xinyong Liu 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-08-26 , DOI: 10.1111/cbdd.13373 Zhao Wang 1 , Dongwei Kang 1 , Meng Chen 2 , Gaochan Wu 1 , Da Feng 1 , Tong Zhao 1 , Zhongxia Zhou 1 , Zhipeng Huo 1 , Lanlan Jing 1 , Xiaofang Zuo 1 , Dirk Daelemans 3 , Erik De Clercq 3 , Christophe Pannecouque 3 , Peng Zhan 1 , Xinyong Liu 1
Affiliation
|
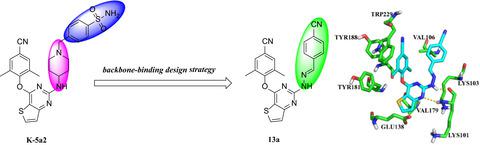
In the previous studies of our laboratory, the thiophene[3,2‐d]pyrimidine was identified as a promising scaffold for seeking highly potent HIV‐1 non‐nucleoside reverse transcriptase inhibitors (NNRTIs). In this study, we designed, synthesized, and biologically evaluated a series of thiophene[3,2‐d]pyrimidine derivatives with changed linker between the thiophenepyrimidine core and the right wing. Some of the synthesized compounds exhibited excellent HIV‐1 inhibitory potency with low (double‐digit) nanomolar 50% effective concentration (EC50) values. Among them, compound 13a exhibited the most potent anti‐HIV‐1 activity (EC50 = 21.2 nM), which was 10‐fold greater than that of NVP (EC50 = 281 nM). Moreover, 13a showed much lower cytotoxicity (CC50 = 183 μM) and higher selection index (SI = 8,632) than NVP, ETV, and AZT. Besides, some physicochemical properties and water solubility were calculated or measured. The preliminary structure–activity relationships and molecular simulation studies of these compounds were also discussed comprehensively to provide valuable direction for further design and optimization.
中文翻译:

新型取代的噻吩[3,2-d]嘧啶衍生物作为有效的人类免疫缺陷病毒-1抑制剂的设计,合成和抗病毒评估
在我们实验室的先前研究中,噻吩[3,2- d ]嘧啶被认为是寻找有力的HIV-1非核苷逆转录酶抑制剂(NNRTIs)的有前途的支架。在这项研究中,我们设计,合成和生物学评估了一系列噻吩[3,2- d ]嘧啶衍生物,并在噻吩嘧啶核心和右翼之间改变了连接子。一些合成的化合物显示出极佳的HIV-1抑制能力,并具有低(两位数)纳摩尔浓度的50%有效浓度(EC 50)值。其中,化合物13a表现出最强的抗HIV-1活性(EC 50 = 21.2 nM),比NVP(EC 50)高10倍。 = 281 nM)。此外, 与NVP,ETV和AZT相比,13a具有更低的细胞毒性(CC 50 = 183μM)和更高的选择指数(SI = 8,632)。此外,还计算或测量了一些理化性质和水溶性。还对这些化合物的初步构效关系和分子模拟研究进行了全面讨论,为进一步设计和优化提供了有价值的指导。
更新日期:2018-08-26
中文翻译:

新型取代的噻吩[3,2-d]嘧啶衍生物作为有效的人类免疫缺陷病毒-1抑制剂的设计,合成和抗病毒评估
在我们实验室的先前研究中,噻吩[3,2- d ]嘧啶被认为是寻找有力的HIV-1非核苷逆转录酶抑制剂(NNRTIs)的有前途的支架。在这项研究中,我们设计,合成和生物学评估了一系列噻吩[3,2- d ]嘧啶衍生物,并在噻吩嘧啶核心和右翼之间改变了连接子。一些合成的化合物显示出极佳的HIV-1抑制能力,并具有低(两位数)纳摩尔浓度的50%有效浓度(EC 50)值。其中,化合物13a表现出最强的抗HIV-1活性(EC 50 = 21.2 nM),比NVP(EC 50)高10倍。 = 281 nM)。此外, 与NVP,ETV和AZT相比,13a具有更低的细胞毒性(CC 50 = 183μM)和更高的选择指数(SI = 8,632)。此外,还计算或测量了一些理化性质和水溶性。还对这些化合物的初步构效关系和分子模拟研究进行了全面讨论,为进一步设计和优化提供了有价值的指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号