Drug Discovery Today ( IF 6.5 ) Pub Date : 2018-08-04 , DOI: 10.1016/j.drudis.2018.08.003 Paolo Rocco , Umberto M. Musazzi , Silvia Franzè , Paola Minghetti
|
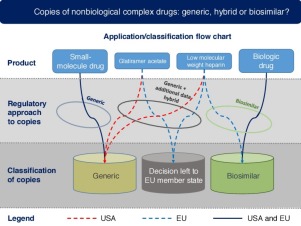
The experience gained with biosimilars has made it clear that copies of complex drugs are more challenging to produce and put on the market than generics. In the case of so-called nonbiological complex drugs (NBCDs), the complexity can arise either from a complex active substance or by other factors, such as formulation or route of delivery. Regulatory policies in the USA and the EU for the marketing of NBCD copies are reviewed, using glatiramer acetate copies as a case study. In the USA, they are approved and marketed as generics (although needing additional data), and so they are interchangeable with the originator. In the EU, they are managed with a hybrid application, and their interchangeability and substitution are established by individual member states.
中文翻译:

非生物复杂药物的副本:通用,混合或生物仿制药?
生物仿制药获得的经验清楚地表明,与仿制药相比,复杂药物的复制品在生产和投放市场方面更具挑战性。就所谓的非生物复合药物(NBCD)而言,复杂性可能是由复杂的活性物质引起的,也可能是由其他因素(例如制剂或递送途径)引起的。审查了美国和欧盟针对NBCD副本销售的监管政策,并以醋酸格拉替雷为例进行了研究。在美国,它们已被批准并作为仿制药销售(尽管需要更多数据),因此它们可以与原研药互换。在欧盟,它们是通过混合应用程序进行管理的,它们的互换性和替代性由各个成员国确定。









































 京公网安备 11010802027423号
京公网安备 11010802027423号