Electrochemistry Communications ( IF 4.7 ) Pub Date : 2018-08-04 , DOI: 10.1016/j.elecom.2018.08.001 Haesol Kim , Min Wook Chung , Chang Hyuck Choi
|
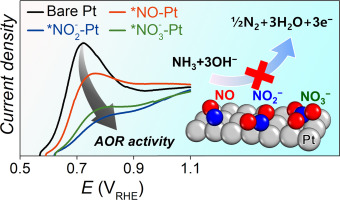
An understanding of the deactivation mechanism of Pt electrocatalysis towards the ammonia oxidation reaction (AOR) to dinitrogen is key to the successful introduction of the nitrogen energy cycle, which can be used as a source of hydrogen fuels. Herein, we study AOR electrocatalysis on NOx-adsorbed polycrystalline Pt electrodes, i.e. NO, NO2− and NO3−, and find that all the NOx species can significantly reduce the catalytic activity of Pt. Combined stationary/transient voltammograms reveal that the poisonous NOx species are produced by NH3 oxidation on the bare Pt surface, but the adsorbed NOx species can be transformed to N2 by the Langmuir–Hinshelwood mechanism with *NO as a key intermediate.
中文翻译:

NO x诱导的Pt电催化氨氧化反应失活
了解Pt电催化氨氧化反应(AOR)生成二氮的失活机理,是成功引入氮能循环的关键,该循环可用作氢燃料。在此,我们研究AOR电催化的NO X -adsorbed多晶铂电极,即NO,NO 2 -和NO 3 - ,发现所有的NO X种可以显著降低铂的催化活性。合并的静止/短暂伏安揭示出有毒NO X由NH产生物种3氧化裸铂表面上,但NO吸附X物种可以转化为N2由Langmuir-Hinshelwood机理研究,其中* NO为关键中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号