Ophthalmology ( IF 13.1 ) Pub Date : 2018-08-03 , DOI: 10.1016/j.ophtha.2018.06.017 Robert N. Weinreb , Jeffrey M. Liebmann , George A. Cioffi , Ivan Goldberg , James D. Brandt , Chris A. Johnson , Linda M. Zangwill , Susan Schneider , Hanh Badger , Marina Bejanian
|
Purpose
To evaluate the effectiveness and safety of oral memantine as a potential neuroprotective agent in open-angle glaucoma (OAG) at risk for progression.
Design
Two randomized, double-masked, placebo-controlled, parallel-group, multicenter, 48-month studies identically designed, initiated 1 year apart, and completed in 2006. Protocol amendments included a 1-year extension (first study) and change in primary endpoint and analysis (second study).
Participants
Patients (2298 total) with bilateral OAG; glaucomatous optic disc damage and visual field loss in 1 eye; glaucomatous optic disc damage and/or visual field loss in the contralateral eye (at screening), topically treated or untreated intraocular pressure (IOP) of 21 mmHg or less (at baseline); and at risk of glaucomatous progression (per prespecified criteria).
Methods
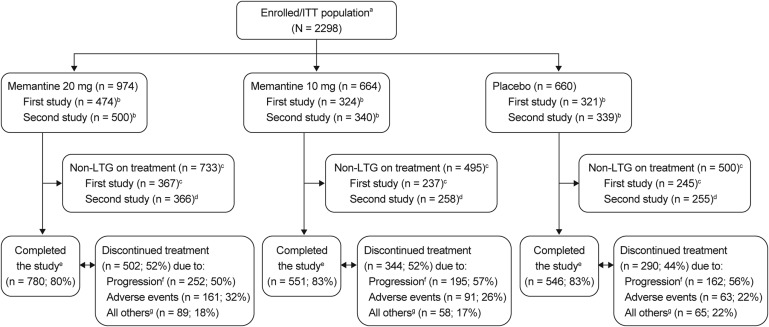
Patients were randomized 3:2:2 to receive memantine 20 mg, memantine 10 mg, or placebo tablets daily. Glaucomatous progression was assessed in the intent-to-treat population by full-threshold standard automated perimetry (SAP), frequency doubling technology (FDT), and stereoscopic optic disc photographs, standardized by quality control assessment at centralized reading centers. Safety evaluations included adverse events (AEs), best-corrected visual acuity, biomicroscopy, IOP, and ophthalmoscopy. Efficacy data from each study were analyzed per protocol. Pooled analyses of efficacy and safety data were also performed.
Main Outcome Measures
The predefined primary efficacy measure was glaucomatous visual field progression, as measured by SAP. Additional efficacy measures included glaucomatous progression of visual field (FDT) and optic nerve damage (stereoscopic optic disc photographs).
Results
The proportion of patients who completed the studies was similar among groups (80%–83%). Compared with placebo, daily treatment with memantine 10 mg or 20 mg for 48 months did not delay glaucomatous progression significantly in the individual studies and pooled analyses. The pooled risk reduction ratio (95% confidence interval) assessed by SAP was −0.13 (−0.40, 0.09) and −0.17 (−0.46, 0.07) for memantine 10 mg and 20 mg, respectively. Results were similar per FDT and stereoscopic optic disc photographs. The most common AEs leading to treatment discontinuations were dizziness, headache, fatigue, and nausea.
Conclusions
With technologies available when the studies were conducted, daily treatment with memantine over 48 months was not shown to prevent glaucomatous progression in this patient population.
中文翻译:

口服美金刚治疗青光眼
目的
评估口服美金刚作为开角型青光眼(OAG)中潜在的神经保护剂的风险,以评估其有效性和安全性。
设计
两项设计相同的随机,双掩蔽,安慰剂对照,平行组,多中心,48个月研究,间隔1年开始,于2006年完成。方案修订包括1年延期(首次研究)和原发性改变终点和分析(第二项研究)。
参加者
双侧OAG患者(共2298例);1只眼的青光眼性视盘损伤和视野丧失;对侧眼青光眼视盘损伤和/或视野丧失(在筛查时),局部治疗或未经治疗的眼内压(IOP)为21 mmHg或更低(在基线时);并有青光眼恶化的风险(按照预先确定的标准)。
方法
患者被随机分为3:2:2每天接受美金刚20 mg,美金刚10 mg或安慰剂片剂。通过全阈值标准自动视野检查(SAP),倍频技术(FDT)和立体视盘照片对意向性治疗人群的青光眼进展进行了评估,并通过集中阅读中心的质量控制评估对其进行了标准化。安全性评估包括不良事件(AE),最佳矫正视力,生物显微镜,眼压和检眼镜。每个研究方案的疗效数据均按协议进行了分析。还对功效和安全性数据进行了汇总分析。
主要观察指标
预先定义的主要疗效指标是青光眼视野进展,如SAP所测量。其他功效指标包括视野青光眼进展(FDT)和视神经损害(立体视盘照片)。
结果
组间完成研究的患者比例相似(80%–83%)。与安慰剂相比,美金刚10 mg或20 mg美金刚的每日治疗48个月在单独的研究和汇总分析中并未显着延迟青光眼的进展。SAP评估的美金刚胺10 mg和20 mg的合并风险降低率(95%置信区间)分别为-0.13(-0.40,0.09)和-0.17(-0.46,0.07)。FDT和立体视盘照片的结果相似。导致治疗中断的最常见AE是头晕,头痛,疲劳和恶心。
结论
进行研究时,利用现有技术,未显示在48个月内每日使用美金刚治疗可预防该患者人群的青光眼进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号