当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand‐Free Bioinspired Suzuki–Miyaura Coupling Reactions using Aryltrifluoroborates as Effective Partners in Deep Eutectic Solvents
ChemSusChem ( IF 7.5 ) Pub Date : 2018-09-05 , DOI: 10.1002/cssc.201801382 Giuseppe Dilauro 1 , Sergio Mata García 2 , Donato Tagarelli 1 , Paola Vitale 1 , Filippo M. Perna 1 , Vito Capriati 1
ChemSusChem ( IF 7.5 ) Pub Date : 2018-09-05 , DOI: 10.1002/cssc.201801382 Giuseppe Dilauro 1 , Sergio Mata García 2 , Donato Tagarelli 1 , Paola Vitale 1 , Filippo M. Perna 1 , Vito Capriati 1
Affiliation
|
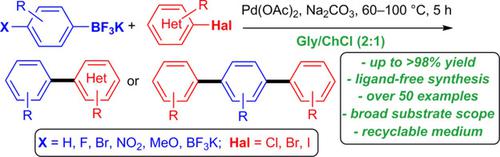
Pd‐catalyzed Suzuki–Miyaura cross‐coupling between (hetero)aryl halides (Cl, Br, I) and versatile, moisture‐stable mono‐ and bifunctional potassium aryltrifluoroborates proceeded efficiently and chemoselectively in air and under generally mild conditions; a catalyst loading as low as 1 mol % combined with Na2CO3 as a base in choline chloride/glycerol (1:2) deep eutectic solvent (DES) was used as a sustainable and environmentally responsible medium. The catalyst, base, and DES were easily and successfully recycled up to six times with an E‐factor as low as 8.74. Valuable biaryls and terphenyl derivatives were furnished in yields of up to 98 %; over 50 reactions were compared and discussed. The methodology was applied for the synthesis of the nonsteroidal anti‐inflammatory drugs Felbinac and Diflunisal.
中文翻译:

芳基三氟硼酸酯作为有效共配物在深共晶溶剂中的无配体生物启发的Suzuki-Miyaura偶联反应
钯催化的(杂)芳基卤化物(Cl,Br,I)与通用的,湿气稳定的单和双官能芳基三氟硼酸钾之间的钯催化的Suzuki-Miyaura交叉偶联在空气中和一般在温和的条件下有效且化学选择性地进行;与Na 2 CO 3结合的催化剂负载量低至1 mol%作为氯化胆碱/甘油(1:2)中的碱,低共熔溶剂(DES)被用作可持续和对环境负责的介质。催化剂,碱和DES可以轻松且成功地回收多达六次,而E因子低至8.74。提供了有价值的联芳基和三联苯衍生物,产率高达98%。比较和讨论了50多个反应。该方法已用于非甾体抗炎药Felbinac和Diflunisal的合成。
更新日期:2018-09-05
中文翻译:

芳基三氟硼酸酯作为有效共配物在深共晶溶剂中的无配体生物启发的Suzuki-Miyaura偶联反应
钯催化的(杂)芳基卤化物(Cl,Br,I)与通用的,湿气稳定的单和双官能芳基三氟硼酸钾之间的钯催化的Suzuki-Miyaura交叉偶联在空气中和一般在温和的条件下有效且化学选择性地进行;与Na 2 CO 3结合的催化剂负载量低至1 mol%作为氯化胆碱/甘油(1:2)中的碱,低共熔溶剂(DES)被用作可持续和对环境负责的介质。催化剂,碱和DES可以轻松且成功地回收多达六次,而E因子低至8.74。提供了有价值的联芳基和三联苯衍生物,产率高达98%。比较和讨论了50多个反应。该方法已用于非甾体抗炎药Felbinac和Diflunisal的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号