Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Systemic siRNA delivery to tumors by cell-penetrating α-helical polypeptide-based metastable nanoparticles†
Nanoscale ( IF 5.8 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1039/c8nr03976c Yang Liu 1, 2, 3 , Ziyuan Song 1, 2, 3 , Nan Zheng 1, 2, 3 , Kenya Nagasaka 2, 3, 4 , Lichen Yin 5, 6, 7, 8, 9 , Jianjun Cheng 1, 2, 3, 5, 6
Nanoscale ( IF 5.8 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1039/c8nr03976c Yang Liu 1, 2, 3 , Ziyuan Song 1, 2, 3 , Nan Zheng 1, 2, 3 , Kenya Nagasaka 2, 3, 4 , Lichen Yin 5, 6, 7, 8, 9 , Jianjun Cheng 1, 2, 3, 5, 6
Affiliation
|
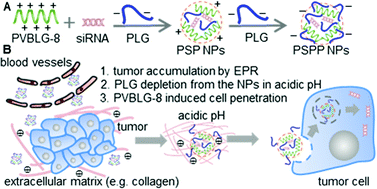
Systemic, non-viral siRNA delivery for cancer treatment is mainly achieved via condensation by cationic materials (e.g., lipids and cationic polymers), which nevertheless, suffers from poor serum stability, non-specific tissue interaction, and unsatisfactory membrane activity against efficient in vivo gene knockdown. Here, we report the design of a metastable, cancer-targeting siRNA delivery system based on two functional polymers, PVBLG-8, a cationic, helical cell-penetrating polypeptide, and poly(L-glutamic acid) (PLG), an anionic random-coiled polypeptide. PVBLG-8 with rigid, linear structure showed weak siRNA condensation capability, and PLG with flexible chains was incorporated as a stabilizer which provided sufficient molecular entanglement with PVBLG-8 to encapsulate the siRNA within the polymeric network. The obtained PVBLG-8/siRNA/PLG nanoparticles (PSP NPs) with positive charges were sequentially coated with additional amount of PLG, which reversed the surface charge from positive to negative to yield the metastable PVBLG-8/siRNA/PLG@PLG (PSPP) NPs. The PSPP NPs featured desired serum stability during circulation to enhance tumor accumulation via the enhanced permeability and retention (EPR) effect. Upon acidification in the tumor extracellular microenvironment and intracellular endosomes, the partial protonation of PLG on PSPP NPs surface would lead to dissociation of PLG coating from NPs, exposure of the highly membrane-active PVBLG-8, and surface charge reversal from negative to positive, which subsequently promoted tumor penetration, selective cancer cell internalization, and efficient endolysosomal escape. When siRNA against epidermal growth factor receptor (EGFR) was encapsulated, the PSPP NPs showed excellent tumor penetration capability, tumor cell uptake level, EGFR silencing efficiency, and tumor growth inhibition efficacy in U-87 MG glioblastoma tumor spheroids in vitro and in xenograft tumor-bearing mice in vivo, outperforming the PSP NPs and several commercial reagents such as Lipofectamine 2000 and poly(L-lysine) (PLL). This study therefore demonstrates a facile and unique design approach of metastable and charge reversal NPs, which overcomes multiple biological barriers against systemic siRNA delivery toward anti-cancer treatment.
中文翻译:

通过细胞穿透的基于α-螺旋多肽的亚稳态纳米颗粒将系统性siRNA递送至肿瘤†
用于癌症治疗的全身性,非病毒性siRNA递送主要是通过阳离子物质(例如脂质和阳离子聚合物)的缩合来实现的,尽管如此,这些物质仍存在血清稳定性差,非特异性组织相互作用以及膜对有效的体内活性不满意的缺点。基因敲低。在这里,我们报告基于两个功能聚合物PVBLG-8,阳离子,螺旋细胞穿透多肽和poly(L-谷氨酸)(PLG),一种阴离子无规卷曲多肽。具有刚性,线性结构的PVBLG-8显示出较弱的siRNA缩合能力,并引入了具有柔性链的PLG作为稳定剂,该稳定剂提供了与PVBLG-8足够的分子缠结,从而将siRNA封装在聚合物网络中。将获得的带正电荷的PVBLG-8 / siRNA / PLG纳米颗粒(PSP NPs)依次涂以额外量的PLG,将表面电荷从正电荷反转为负电荷,以产生亚稳的PVBLG-8 / siRNA / PLG @ PLG(PSPP )NP。PSPP NPs在循环过程中具有所需的血清稳定性,可通过以下途径增强肿瘤蓄积增强的渗透性和保留(EPR)效果。在肿瘤细胞外微环境和细胞内内体中酸化后,PSPP NPs表面PLG的部分质子化将导致PLG涂层从NP上解离,暴露出具有高膜活性的PVBLG-8,并且表面电荷从负转为正,随后促进了肿瘤的渗透,选择性的癌细胞内在化和有效的溶酶体逃逸。当siRNA的抗表皮生长因子受体(EGFR)包封,所述PSPP的NP表现出优异的肿瘤穿透能力,肿瘤细胞摄取水平,EGFR沉默效率,并且以U-87 MG成胶质细胞瘤肿瘤球状体的肿瘤生长抑制效力的体外和在异种移植肿瘤体内小鼠胜过PSP NP和几种商用试剂,如Lipofectamine 2000和聚L-赖氨酸(PLL)。因此,本研究证明了亚稳态和电荷逆转NP的简便且独特的设计方法,该方法克服了针对全身siRNA传递抗癌治疗的多种生物学障碍。
更新日期:2018-08-02
中文翻译:

通过细胞穿透的基于α-螺旋多肽的亚稳态纳米颗粒将系统性siRNA递送至肿瘤†
用于癌症治疗的全身性,非病毒性siRNA递送主要是通过阳离子物质(例如脂质和阳离子聚合物)的缩合来实现的,尽管如此,这些物质仍存在血清稳定性差,非特异性组织相互作用以及膜对有效的体内活性不满意的缺点。基因敲低。在这里,我们报告基于两个功能聚合物PVBLG-8,阳离子,螺旋细胞穿透多肽和poly(L-谷氨酸)(PLG),一种阴离子无规卷曲多肽。具有刚性,线性结构的PVBLG-8显示出较弱的siRNA缩合能力,并引入了具有柔性链的PLG作为稳定剂,该稳定剂提供了与PVBLG-8足够的分子缠结,从而将siRNA封装在聚合物网络中。将获得的带正电荷的PVBLG-8 / siRNA / PLG纳米颗粒(PSP NPs)依次涂以额外量的PLG,将表面电荷从正电荷反转为负电荷,以产生亚稳的PVBLG-8 / siRNA / PLG @ PLG(PSPP )NP。PSPP NPs在循环过程中具有所需的血清稳定性,可通过以下途径增强肿瘤蓄积增强的渗透性和保留(EPR)效果。在肿瘤细胞外微环境和细胞内内体中酸化后,PSPP NPs表面PLG的部分质子化将导致PLG涂层从NP上解离,暴露出具有高膜活性的PVBLG-8,并且表面电荷从负转为正,随后促进了肿瘤的渗透,选择性的癌细胞内在化和有效的溶酶体逃逸。当siRNA的抗表皮生长因子受体(EGFR)包封,所述PSPP的NP表现出优异的肿瘤穿透能力,肿瘤细胞摄取水平,EGFR沉默效率,并且以U-87 MG成胶质细胞瘤肿瘤球状体的肿瘤生长抑制效力的体外和在异种移植肿瘤体内小鼠胜过PSP NP和几种商用试剂,如Lipofectamine 2000和聚L-赖氨酸(PLL)。因此,本研究证明了亚稳态和电荷逆转NP的简便且独特的设计方法,该方法克服了针对全身siRNA传递抗癌治疗的多种生物学障碍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号