当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-assembly of tetra-nuclear lanthanide clusters via atmospheric CO2 fixation: interesting solvent-induced structures and magnetic relaxation conversions†
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1039/c8qi00573g Wen-Min Wang 1, 2, 3, 4, 5 , Zhi-Lei Wu 1, 2, 3, 4, 5 , Ya-Xin Zhang 1, 2, 3, 4 , Hai-Ying Wei 1, 2, 3, 4 , Hong-Ling Gao 6, 7, 8, 9 , Jian-Zhong Cui 6, 7, 8, 9
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1039/c8qi00573g Wen-Min Wang 1, 2, 3, 4, 5 , Zhi-Lei Wu 1, 2, 3, 4, 5 , Ya-Xin Zhang 1, 2, 3, 4 , Hai-Ying Wei 1, 2, 3, 4 , Hong-Ling Gao 6, 7, 8, 9 , Jian-Zhong Cui 6, 7, 8, 9
Affiliation
|
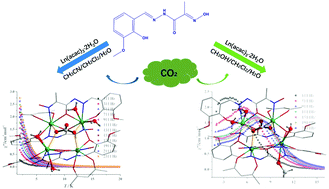
Two systems of carbonate complexes were constructed by employing a newly prepared Schiff based ligand (H2L; H2L = 2-(hydroxyimino)-2-[(3-methoxyl-2-hydroxyphenyl)methylene]hydrazide) and Ln(acac)3·H2O (Hacac = acetylacetone) in different reaction solutions, namely [Ln4(CO3)(L)4(acac)2(H2O)4]·2CH3CN (Ln = Gd(1), Dy(2)) and [Ln4(CO3)(L)4(acac)2(CH3OH)2(H2O)2]·CH3OH·H2O (Ln = Gd(3), Dy(4)). Interestingly, complexes 1–4 are nearly structurally identical except for the coordinated solvent molecules. All of them consist of a Ln4 cluster in the center, in which four Ln(III) ions are bridged by two μ2-O atoms from the in situ formed CO32− through spontaneous fixation of atmospheric CO2. Magnetic investigation suggests that complexes 1 and 3 display magnetic refrigeration behaviors with maximum values of magnetic entropy changes of 31.23 J kg−1 K−1 and 27.06 J kg−1 K−1, respectively. Additionally, the energy barriers of the magnetization reversal were significantly improved from 2.73 K for 2 to 23.79 K for 4 by deliberately using methanol to replace the coordinated H2O molecule. It should be noted that the divergence in structures and magnetic properties could be mainly ascribed to the different solvent effects in the synthesis process.
中文翻译:

通过大气CO 2固定作用 自组装四核镧系元素团:有趣的溶剂诱导结构和磁弛豫转换†
通过使用新制备的基于席夫(Schiff)的配体(H 2 L; H 2 L = 2-(羟基亚氨基)-2-[(3-甲氧基-2-羟基苯基)亚甲基]酰肼)和Ln(acac)构造两个碳酸盐配合物体系)3 ·H 2 O(Hacac =乙酰丙酮)在不同的反应溶液中,即[Ln 4(CO 3)(L)4(acac)2(H 2 O)4 ]·2CH 3 CN(Ln = Gd(1) ,Dy(2))和[Ln 4(CO 3)(L)4(acac)2(CH 3 OH)2(H 2 O)2 ]·CH 3 OH·H 2 O(Ln = Gd(3),Dy(4))。有趣的是,配合物1-4除了配位的溶剂分子外,在结构上几乎相同。所有这些由一个LN的4在中心簇,其中四个LN(III)离子被两个μ桥接2 -O原子从原位形成CO 3 2-,通过大气中的二氧化碳的自发固定2。磁性研究表明配合物1和3分别以31.23 J kg -1 K -1和27.06 J kg -1 K -1的最大磁熵变显示磁性制冷行为。此外,通过故意使用甲醇代替配位的H 2 O分子,磁化反转的能垒从2.73 K / 2显着提高到23.79 K / 4。应当指出,结构和磁性能的差异可能主要归因于合成过程中不同的溶剂作用。
更新日期:2018-08-02
中文翻译:

通过大气CO 2固定作用 自组装四核镧系元素团:有趣的溶剂诱导结构和磁弛豫转换†
通过使用新制备的基于席夫(Schiff)的配体(H 2 L; H 2 L = 2-(羟基亚氨基)-2-[(3-甲氧基-2-羟基苯基)亚甲基]酰肼)和Ln(acac)构造两个碳酸盐配合物体系)3 ·H 2 O(Hacac =乙酰丙酮)在不同的反应溶液中,即[Ln 4(CO 3)(L)4(acac)2(H 2 O)4 ]·2CH 3 CN(Ln = Gd(1) ,Dy(2))和[Ln 4(CO 3)(L)4(acac)2(CH 3 OH)2(H 2 O)2 ]·CH 3 OH·H 2 O(Ln = Gd(3),Dy(4))。有趣的是,配合物1-4除了配位的溶剂分子外,在结构上几乎相同。所有这些由一个LN的4在中心簇,其中四个LN(III)离子被两个μ桥接2 -O原子从原位形成CO 3 2-,通过大气中的二氧化碳的自发固定2。磁性研究表明配合物1和3分别以31.23 J kg -1 K -1和27.06 J kg -1 K -1的最大磁熵变显示磁性制冷行为。此外,通过故意使用甲醇代替配位的H 2 O分子,磁化反转的能垒从2.73 K / 2显着提高到23.79 K / 4。应当指出,结构和磁性能的差异可能主要归因于合成过程中不同的溶剂作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号