当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free alkene oxy- and amino-perfluoroalkylations via carbocation formation by using perfluoro acid anhydrides: unique reactivity between styrenes and perfluoro diacyl peroxides†
Chemical Science ( IF 7.6 ) Pub Date : 2018-08-01 00:00:00 , DOI: 10.1039/c8sc02547a Elena Valverde 1 , Shintaro Kawamura 1, 2 , Daisuke Sekine 1 , Mikiko Sodeoka 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2018-08-01 00:00:00 , DOI: 10.1039/c8sc02547a Elena Valverde 1 , Shintaro Kawamura 1, 2 , Daisuke Sekine 1 , Mikiko Sodeoka 1, 2
Affiliation
|
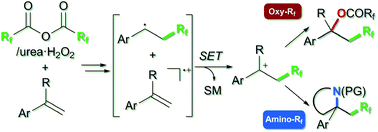
We present a strategy for metal-free, alkene difunctionalization-type, oxy- and amino-perfluoroalkylations, using perfluoro acid anhydrides as practical and user-friendly perfluoroalkyl sources. This method provides efficient access to oxy-perfluoroalkylation products via carbocation formation due to the unique reactivity between styrenes and bis(perfluoroacyl) peroxides generated in situ from perfluoro acid anhydrides. This reaction is also applicable to metal-free intramolecular amino-perfluoroalkylation of styrenes bearing a pendant amino group. Synthetic utility of the oxy-trifluoromethylation products was confirmed by demonstrating derivatization via hydrolysis, elimination, and acid-catalyzed substitution with carbon nucleophiles. The mechanism of the carbocation formation was investigated experimentally and theoretically.
中文翻译:

使用全氟酸酐通过碳阳离子形成无金属烯烃氧和氨基全氟烷基化:苯乙烯和全氟二酰基过氧化物之间的独特反应性†
我们提出了一种无金属、烯烃双官能化型、氧基和氨基全氟烷基化的策略,使用全氟酸酐作为实用且用户友好的全氟烷基源。由于苯乙烯和全氟酸酐原位产生的双(全氟酰基)过氧化物之间的独特反应性,该方法提供了通过碳阳离子形成有效获得氧-全氟烷基化产物的方法。该反应也适用于带有侧氨基的苯乙烯的无金属分子内氨基全氟烷基化。氧三氟甲基化产物的合成效用通过证明衍生化得到证实碳亲核试剂的水解、消除和酸催化取代。对碳正离子形成的机理进行了实验和理论研究。
更新日期:2018-08-01
中文翻译:

使用全氟酸酐通过碳阳离子形成无金属烯烃氧和氨基全氟烷基化:苯乙烯和全氟二酰基过氧化物之间的独特反应性†
我们提出了一种无金属、烯烃双官能化型、氧基和氨基全氟烷基化的策略,使用全氟酸酐作为实用且用户友好的全氟烷基源。由于苯乙烯和全氟酸酐原位产生的双(全氟酰基)过氧化物之间的独特反应性,该方法提供了通过碳阳离子形成有效获得氧-全氟烷基化产物的方法。该反应也适用于带有侧氨基的苯乙烯的无金属分子内氨基全氟烷基化。氧三氟甲基化产物的合成效用通过证明衍生化得到证实碳亲核试剂的水解、消除和酸催化取代。对碳正离子形成的机理进行了实验和理论研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号