当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Active C8-Acyl-ACP Thioesterase Variant Isolated by a Synthetic Selection Strategy
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-07-31 00:00:00 , DOI: 10.1021/acssynbio.8b00215 Néstor J. Hernández Lozada 1 , Rung-Yi Lai 1 , Trevor R. Simmons 1 , Kelsey A. Thomas 1 , Ratul Chowdhury 2 , Costas D. Maranas 2 , Brian F. Pfleger 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-07-31 00:00:00 , DOI: 10.1021/acssynbio.8b00215 Néstor J. Hernández Lozada 1 , Rung-Yi Lai 1 , Trevor R. Simmons 1 , Kelsey A. Thomas 1 , Ratul Chowdhury 2 , Costas D. Maranas 2 , Brian F. Pfleger 1
Affiliation
|
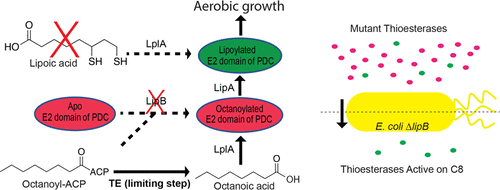
Microbial metabolism is an attractive route for producing medium chain length fatty acids, e.g., octanoic acid, used in the oleochemical industry. One challenge to this strategy is the lack of enzymes that are both highly active in a microbial host and selective toward substrates with desired chain length. Of the many steps in fatty acid biosynthesis, the thioesterase is the most widely used enzyme for controlling chain length. Thioesterases hydrolyze the thioester bond between fatty acids and the acyl-carrier protein (ACP) or coenzyme A (CoA) cofactor. The functional role of thioesterases varies between organisms (i.e., bacteria vs plant) and therefore so do the substrate specificities. As a result, microbial biocatalysts that utilize a heterologous thioesterase either produce high titers of fatty acids with mixed chain lengths or low titers of products with a narrow chain length distribution. To search for highly active enzymes that selectively hydrolyze octanoyl-ACP, we developed a genetic selection based on the lipoic acid requirement of Escherichia coli. We used the selection to identify variants in a randomly mutagenized library of the C8-specific Cuphea palustris FatB1 thioesterase. After optimizing expression of the thioesterase, E. coli cultures produced 1.7 g/L of octanoic acid with >90% specificity from a single chromosomal copy of this thioesterase. In vitro studies confirmed the mutant thioesterase possessed a 15-fold increase in kcat compared to its native sequence. The high level of specific activity allowed for low levels of expression while maintaining fatty acid titer. The low expression requirement will allow metabolic engineers to use more cellular resources to address other limitations in the pathway and maximize overall productivity.
中文翻译:

通过合成选择策略分离的高活性C 8-酰基-ACP硫酯酶变体
微生物代谢是生产在油脂化学工业中使用的中等链长脂肪酸例如辛酸的有吸引力的途径。该策略的挑战之一是缺乏既在微生物宿主中具有高活性又对具有所需链长的底物具有选择性的酶。在脂肪酸生物合成的许多步骤中,硫酯酶是控制链长最广泛使用的酶。硫酯酶水解脂肪酸与酰基载体蛋白(ACP)或辅酶A(CoA)辅因子之间的硫酯键。硫酯酶的功能作用因生物体而异(即细菌与植物),因此底物特异性也是如此。结果,利用异源硫酯酶的微生物生物催化剂要么产生高滴度的具有混合链长的脂肪酸,要么产生低滴度的具有窄链长分布的产物。为了寻找可选择性水解辛酰ACP的高活性酶,我们根据大肠杆菌的硫辛酸需求进行了遗传选择。我们使用的选择,以确定在C的随机诱变库变体8特异性萼距沼泽FatB1硫酯酶。优化硫酯酶的表达后,大肠杆菌培养物从该硫酯酶的单个染色体拷贝中产生了1.7 g / L的辛酸,其特异性> 90%。体外研究证实,与其天然序列相比,突变型硫酯酶的k cat含量增加了15倍。高水平的比活性允许低水平的表达,同时保持脂肪酸滴度。低表达要求将使新陈代谢工程师能够使用更多的细胞资源来解决该途径中的其他限制,并最大限度地提高整体生产率。
更新日期:2018-07-31
中文翻译:

通过合成选择策略分离的高活性C 8-酰基-ACP硫酯酶变体
微生物代谢是生产在油脂化学工业中使用的中等链长脂肪酸例如辛酸的有吸引力的途径。该策略的挑战之一是缺乏既在微生物宿主中具有高活性又对具有所需链长的底物具有选择性的酶。在脂肪酸生物合成的许多步骤中,硫酯酶是控制链长最广泛使用的酶。硫酯酶水解脂肪酸与酰基载体蛋白(ACP)或辅酶A(CoA)辅因子之间的硫酯键。硫酯酶的功能作用因生物体而异(即细菌与植物),因此底物特异性也是如此。结果,利用异源硫酯酶的微生物生物催化剂要么产生高滴度的具有混合链长的脂肪酸,要么产生低滴度的具有窄链长分布的产物。为了寻找可选择性水解辛酰ACP的高活性酶,我们根据大肠杆菌的硫辛酸需求进行了遗传选择。我们使用的选择,以确定在C的随机诱变库变体8特异性萼距沼泽FatB1硫酯酶。优化硫酯酶的表达后,大肠杆菌培养物从该硫酯酶的单个染色体拷贝中产生了1.7 g / L的辛酸,其特异性> 90%。体外研究证实,与其天然序列相比,突变型硫酯酶的k cat含量增加了15倍。高水平的比活性允许低水平的表达,同时保持脂肪酸滴度。低表达要求将使新陈代谢工程师能够使用更多的细胞资源来解决该途径中的其他限制,并最大限度地提高整体生产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号