当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Enrichment/Enzymatic Approach To Study Tightly Clustered Multisite Phosphorylation on Ser-Rich Domains
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-08-09 , DOI: 10.1021/acs.jproteome.8b00205 Evgeny Kanshin , Mirela Pascariu , Mike Tyers , Damien D’Amours 1 , Pierre Thibault
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-08-09 , DOI: 10.1021/acs.jproteome.8b00205 Evgeny Kanshin , Mirela Pascariu , Mike Tyers , Damien D’Amours 1 , Pierre Thibault
Affiliation
|
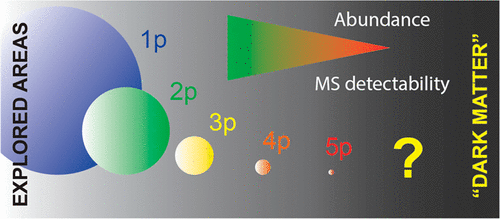
The regulation of protein function through phosphorylation is often dominated by allosteric interactions and conformational changes. However, alternative mechanisms involving electrostatic interactions also regulate protein function. In particular, phosphorylation of clusters of Ser/Thr residues can affect protein-plasma membrane/chromatin interactions by electrostatic interactions between phosphosites and phospholipids or histones. Currently, only a few examples of such mechanisms are reported, primarily because of the difficulties of detecting highly phosphorylated proteins and peptides, due in part to the low ionization efficiency and fragmentation yield of multiphosphorylated peptides in mass spectrometry when using positive ion mode detection. This difficulty in detection has resulted in under-reporting of such modified regions, which can be thought of as phosphoproteomic dark matter. Here, we present a novel approach that enriches for multisite-phosphorylated peptides that until now remained inaccessible by conventional phosphoproteomics. Our technique enables the identification of multisite-phosphorylated regions on more than 300 proteins in both yeast and human cells and can be used to profile changes in multisite phosphorylation upon cell stimulation. We further characterize the role of multisite phosphorylation for Ste20 in the yeast mating pheromone response. Mutagenesis experiments confirmed that multisite phosphorylation of Ser/Thr-rich regions plays an important role in the regulation of Ste20 activity during mating pheromone signaling. The ability to detect protein multisite phosphorylation opens new avenues to explore phosphoproteomic dark matter and to study Ser-rich proteins that interact with binding partners through charge pairing mechanisms.
中文翻译:

富集/酶结合方法研究富含Ser的域上紧密簇聚的多位磷酸化
通过磷酸化对蛋白质功能的调节通常由变构相互作用和构象变化决定。但是,涉及静电相互作用的其他机制也调节蛋白质功能。特别地,Ser / Thr残基簇的磷酸化可通过磷酸位与磷脂或组蛋白之间的静电相互作用影响蛋白质-质膜/染色质相互作用。目前,仅报道了这种机制的几个例子,主要是由于难以检测高度磷酸化的蛋白质和肽段,部分原因是在质谱法中使用正离子模式检测时,多磷酸化的肽段的电离效率低且碎片化率高。这种检测上的困难导致此类修饰区域的报告不足,可以认为是磷酸蛋白质组学的暗物质。在这里,我们提出了一种新颖的方法,该方法可富集多位磷酸化的肽,该肽到现在为止仍是常规磷酸蛋白组学无法获得的。我们的技术能够识别酵母和人类细胞中300多种蛋白质上的多位磷酸化区域,并可用于分析细胞刺激后多位磷酸化的变化。我们进一步表征在酵母交配信息素反应中Ste20的多位磷酸化的作用。诱变实验证实,富含Ser / Thr的区域的多位磷酸化在信息素交配过程中对Ste20活性的调节中起着重要作用。
更新日期:2018-08-10
中文翻译:

富集/酶结合方法研究富含Ser的域上紧密簇聚的多位磷酸化
通过磷酸化对蛋白质功能的调节通常由变构相互作用和构象变化决定。但是,涉及静电相互作用的其他机制也调节蛋白质功能。特别地,Ser / Thr残基簇的磷酸化可通过磷酸位与磷脂或组蛋白之间的静电相互作用影响蛋白质-质膜/染色质相互作用。目前,仅报道了这种机制的几个例子,主要是由于难以检测高度磷酸化的蛋白质和肽段,部分原因是在质谱法中使用正离子模式检测时,多磷酸化的肽段的电离效率低且碎片化率高。这种检测上的困难导致此类修饰区域的报告不足,可以认为是磷酸蛋白质组学的暗物质。在这里,我们提出了一种新颖的方法,该方法可富集多位磷酸化的肽,该肽到现在为止仍是常规磷酸蛋白组学无法获得的。我们的技术能够识别酵母和人类细胞中300多种蛋白质上的多位磷酸化区域,并可用于分析细胞刺激后多位磷酸化的变化。我们进一步表征在酵母交配信息素反应中Ste20的多位磷酸化的作用。诱变实验证实,富含Ser / Thr的区域的多位磷酸化在信息素交配过程中对Ste20活性的调节中起着重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号