当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Implication of Water on Fragment‐to‐Ligand Growth in Kinase Binding Thermodynamics
ChemMedChem ( IF 3.6 ) Pub Date : 2018-08-23 , DOI: 10.1002/cmdc.201800438 Barbara Wienen-Schmidt 1 , Tobias Wulsdorf 1 , Hendrik R. A. Jonker 2 , Krishna Saxena 2 , Denis Kudlinzki 2 , Verena Linhard 2 , Sridhar Sreeramulu 2 , Andreas Heine 1 , Harald Schwalbe 2 , Gerhard Klebe 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-08-23 , DOI: 10.1002/cmdc.201800438 Barbara Wienen-Schmidt 1 , Tobias Wulsdorf 1 , Hendrik R. A. Jonker 2 , Krishna Saxena 2 , Denis Kudlinzki 2 , Verena Linhard 2 , Sridhar Sreeramulu 2 , Andreas Heine 1 , Harald Schwalbe 2 , Gerhard Klebe 1
Affiliation
|
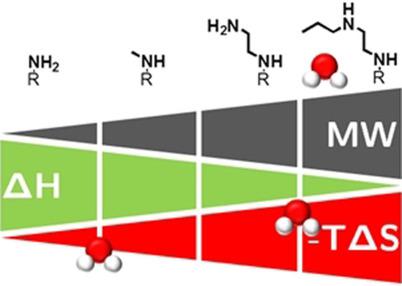
A ligand‐binding study is presented focusing on thermodynamics of fragment expansion. The binding of four compounds with increasing molecular weight to protein kinase A (PKA) was analyzed. The ligands display affinities between low‐micromolar to nanomolar potency despite their low molecular weight. Binding free energies were measured by isothermal titration calorimetry, revealing a trend toward more entropic and less enthalpic binding with increase in molecular weight. All protein–ligand complexes were analyzed by crystallography and solution NMR spectroscopy. Crystal structures and solution NMR data are highly consistent, and no major differences in complex dynamics across the series are observed that would explain the differences in the thermodynamic profiles. Instead, the thermodynamic trends result either from differences in the solvation patterns of the conformationally more flexible ligand in aqueous solution prior to protein binding as molecular dynamics simulations suggest, or from local shifts of the water structure in the ligand‐bound state. Our data thus provide evidence that changes in the solvation pattern constitute an important parameter for the understanding of thermodynamic data in protein–ligand complex formation.
中文翻译:

水对激酶结合热力学中片段到配体生长的影响
提出了结合配体的研究,其重点是片段扩展的热力学。分析了四种分子量增加的化合物与蛋白激酶A(PKA)的结合。配体尽管分子量低,但仍显示出低微摩尔至纳摩尔的亲和力。通过等温滴定量热法测量结合自由能,揭示随着分子量的增加,熵变和焓变降低的趋势。所有蛋白质-配体复合物均通过晶体学和溶液NMR光谱分析。晶体结构和溶液NMR数据高度一致,并且在整个系列中没有观察到复杂动力学的主要差异,这可以解释热力学曲线的差异。反而,热力学趋势是由于分子动力学模拟表明,蛋白质结合之前在水溶液中构象更灵活的配体在溶剂中的溶剂化方式不同,或者是由于配体结合状态下水结构的局部移动所致。因此,我们的数据提供了证据,表明溶剂化模式的变化是理解蛋白质-配体复合物形成中热力学数据的重要参数。
更新日期:2018-08-23
中文翻译:

水对激酶结合热力学中片段到配体生长的影响
提出了结合配体的研究,其重点是片段扩展的热力学。分析了四种分子量增加的化合物与蛋白激酶A(PKA)的结合。配体尽管分子量低,但仍显示出低微摩尔至纳摩尔的亲和力。通过等温滴定量热法测量结合自由能,揭示随着分子量的增加,熵变和焓变降低的趋势。所有蛋白质-配体复合物均通过晶体学和溶液NMR光谱分析。晶体结构和溶液NMR数据高度一致,并且在整个系列中没有观察到复杂动力学的主要差异,这可以解释热力学曲线的差异。反而,热力学趋势是由于分子动力学模拟表明,蛋白质结合之前在水溶液中构象更灵活的配体在溶剂中的溶剂化方式不同,或者是由于配体结合状态下水结构的局部移动所致。因此,我们的数据提供了证据,表明溶剂化模式的变化是理解蛋白质-配体复合物形成中热力学数据的重要参数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号