当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric polyoxometalate electrolytes for advanced redox flow batteries†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-07-28 00:00:00 , DOI: 10.1039/c8ee00422f Jochen Friedl 1, 2, 3, 4, 5 , Matthäa V. Holland-Cunz 1, 2, 3, 4, 5 , Faye Cording 1, 2, 3, 4, 5 , Felix L. Pfanschilling 1, 2, 3, 4, 5 , Corinne Wills 1, 2, 3, 4, 5 , William McFarlane 1, 2, 3, 4, 5 , Barbara Schricker 6, 7, 8, 9 , Robert Fleck 6, 7, 8, 9 , Holger Wolfschmidt 6, 7, 8, 9 , Ulrich Stimming 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-07-28 00:00:00 , DOI: 10.1039/c8ee00422f Jochen Friedl 1, 2, 3, 4, 5 , Matthäa V. Holland-Cunz 1, 2, 3, 4, 5 , Faye Cording 1, 2, 3, 4, 5 , Felix L. Pfanschilling 1, 2, 3, 4, 5 , Corinne Wills 1, 2, 3, 4, 5 , William McFarlane 1, 2, 3, 4, 5 , Barbara Schricker 6, 7, 8, 9 , Robert Fleck 6, 7, 8, 9 , Holger Wolfschmidt 6, 7, 8, 9 , Ulrich Stimming 1, 2, 3, 4, 5
Affiliation
|
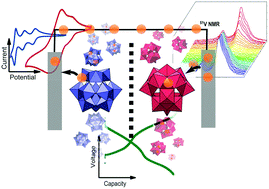
Electrochemical storage of energy is a necessary asset for the integration of intermittent renewable energy sources such as wind and solar power into a complete energy scenario. Redox flow batteries (RFBs) are the only type of battery in which the energy content and the power output can be scaled independently, offering flexibility for applications such as load levelling. However, the prevailing technology, the all Vanadium system, comprises low energy and low power densities. In this study we investigate two polyoxometalates (POMs), [SiW12O40]4− and [PV14O42]9−, as nano-sized electron shuttles. We show that these POMs exhibit fast redox kinetics (electron transfer constant k0 ≈ 10−2 cm s−1 for [SiW12O40]4−), thereby enabling high power densities; in addition, they feature multi-electron transfer, realizing a high capacity per molecule; they do not cross cation exchange membranes, eliminating self-discharge through the separator; and they are chemically and electrochemically stable as shown by in situ NMR. In flow battery studies the theoretical capacity (10.7 A h L−1) could be achieved under operating conditions. The cell was cycled for 14 days with current densities in the range of 30 to 60 mA cm−2 (155 cycles). The Coulombic efficiency was 94% during cycling. Very small losses occurred due to residual oxygen in the system. The voltage efficiency (∼65% at 30 mA cm−2) was mainly affected by ohmic rather than kinetic losses. Pathways for further improvement are discussed.
中文翻译:

用于高级氧化还原液流电池的不对称多金属氧酸盐电解质†
电能的电化学存储是将间歇性可再生能源(例如风能和太阳能)整合到完整能源方案中的必要资产。氧化还原液流电池(RFB)是唯一可以独立调整能量含量和输出功率的电池类型,为负载均衡等应用提供了灵活性。但是,最流行的技术,即全钒系统,具有低能耗和低功率密度。在这项研究中,我们研究了两种多金属氧酸盐(POM)[SiW 12 O 40 ] 4−和[PV 14 O 42 ] 9−,它们是纳米级的电子穿梭体。我们表明,这些POM表现出快速的氧化还原动力学(电子转移常数ķ 0 ≈10 -2厘米小号-1为[硅钨酸12 ø 40 ] 4-),从而使高功率密度; 此外,它们具有多电子转移的特征,可实现每个分子的高容量。它们不穿过阳离子交换膜,从而消除了通过隔板的自放电。并且如原位NMR所示,它们在化学和电化学上都是稳定的。在液流电池研究中,可以在工作条件下达到理论容量(10.7 A h L -1)。电池以30至60 mA cm -2的电流密度循环14天(155个周期)。循环过程中的库仑效率为94%。由于系统中残留有氧气,因此损失很小。电压效率(在30 mA cm -2时约为65%)主要受欧姆而不是动力学损耗的影响。讨论了进一步改进的途径。
更新日期:2018-07-28
中文翻译:

用于高级氧化还原液流电池的不对称多金属氧酸盐电解质†
电能的电化学存储是将间歇性可再生能源(例如风能和太阳能)整合到完整能源方案中的必要资产。氧化还原液流电池(RFB)是唯一可以独立调整能量含量和输出功率的电池类型,为负载均衡等应用提供了灵活性。但是,最流行的技术,即全钒系统,具有低能耗和低功率密度。在这项研究中,我们研究了两种多金属氧酸盐(POM)[SiW 12 O 40 ] 4−和[PV 14 O 42 ] 9−,它们是纳米级的电子穿梭体。我们表明,这些POM表现出快速的氧化还原动力学(电子转移常数ķ 0 ≈10 -2厘米小号-1为[硅钨酸12 ø 40 ] 4-),从而使高功率密度; 此外,它们具有多电子转移的特征,可实现每个分子的高容量。它们不穿过阳离子交换膜,从而消除了通过隔板的自放电。并且如原位NMR所示,它们在化学和电化学上都是稳定的。在液流电池研究中,可以在工作条件下达到理论容量(10.7 A h L -1)。电池以30至60 mA cm -2的电流密度循环14天(155个周期)。循环过程中的库仑效率为94%。由于系统中残留有氧气,因此损失很小。电压效率(在30 mA cm -2时约为65%)主要受欧姆而不是动力学损耗的影响。讨论了进一步改进的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号